Abstract
Introduction. We investigated the roles of melatonin (a powerful antioxidant, iNOS inhibitor, and a scavenger of peroxynitrite) and 1400W (a strong and selective inhibitor of inducible nitric oxide) on renal dysfunction and injury induced by ischemia/reperfusion (I/R) of rat kidney, since oxidative and nitrosative injury are believed to be the major causes. Materials and methods. Thirty-two male Sprague-Dawley rats were divided into four groups of sham-operated, I/R, I/R + Melatonin and I/R + 1400W. Rats were given either melatonin (10 mg/kg) or 1400W (10 mg/kg) in the I/R + Melatonin and I/R + 1400W groups respectively at 6 h prior to ischemia and at the beginning of reperfusion via intraperitoneal route. I/R injury was induced by 60 min of bilateral renal ischemia followed by 6 h of reperfusion. After reperfusion, kidneys and blood were obtained for histopathologic and biochemical evaluation. Results. Melatonin and 1400W had an ameliorative effect on both oxidative and nitrosative stress in the kidneys against renal I/R injury in rats. In addition, melatonin significantly reduced elevated nitro-oxidative stress product, restored decreased antioxidant enzymes and attenuated histological alterations when compared with 1400W. Conclusions. Both Melatonin and 1400W were efficient in ameliorating experimental I/R injury of the kidneys. Moreover, melatonin was more effective than 1400W possibly through inhibiting iNOS as well as scavenging free oxygen radicals and peroxynitrite.
INTRODUCTION
Cessation of kidney blood supply leads to acute renal failure (ARF), causing failure of the kidneys over a period of hours or days.Citation[1–3] The causes of ARF are multifactorial, but ischemic ARF caused by hypotension followed by resuscitation is frequent, the etiology of which is reflected in animal models of renal ischemia/reperfusion (I/R).Citation[1,4]
Reperfusion of the kidneys causes the activation and adhesion of polymorphonuclear neutrophils (PMNL), with the release of proinflammatory substances and the formation of free radicals, which are nitrogen-derived reactive nitrogen species (RNS) or oxygen-derived reactive oxygen species (ROS), such as superoxide (O•2−), hydrogen peroxide (H2O2), and hydroxyl radicals (•OH).Citation[1,4–8]
It is presumed that ROS production reduces the transcription of endothelial nitric oxide synthase (eNOS), which provides nitric oxide (NO) under physiologic circumstances. On the other hand, ROS/RNS activates inducible nitric oxide synthase (iNOS), which causes an almost 1,000-fold higher NO production than eNOS. Intensified expression of iNOS has been detected in virtually all cell types tested, including macrophages, fibroblasts, chondrocytes, osteoclasts, and epithelial cells, and results in the production of large amounts of NO in animals and patients with inflammatory diseases.Citation[9–12] In addition, the level of iNOS expression is well correlated with the degree of inflammation. Once iNOS is activated, because of NO's affinity for O•2−, neither enzymatic nor pharmacologic levels of conventional antioxidants are able to compete with NO for O•2−; as a result, high peroxynitrite (ONOO−) levels are elevated.Citation[5,13,14] Because NO derived from iNOS also has an important role in I/R process, it is crucial to determine whether NO or ONOO− exerted these effects.Citation[7,15] Various in vivo studies have shown that NO biosynthesis and its action are closely related to the pathogenesis of renal I/R injury. In addition, it has been shown that selective or non-selective iNOS inhibitors prevent I/R injury in skeletal muscle, myocardial infarction, brain, liver, and kidney tissues,Citation[13,16–21] and that iNOS–knockout mice show reduced I/R injury in the kidney.Citation[20]
Melatonin is mainly secreted from pineal gland and a variety of non-pineal tissues and organs, including the kidney.Citation[22] Non-pineal melatonin is believed to function as an antioxidant and free radical scavenger within the cells and has been shown to have protective effects against I/R injury in kidney, heart, and intestine, among others.Citation[22–24] Additionally, several of melatonin's metabolites are highly effective radical scavengers.Citation[25,26] N-[3(aminomethyl) benzyl) acetamidine] (1400W) is a highly selective iNOS inhibitor that decreases I/R injury in both kidney and heart in experimental studies.Citation[27,28]
We hypothesized that the melatonin as a strong antioxidant and iNOS inhibitor agent as well as a scavenger of peroxynitrite may counteract more efficiently than 1400W, which is an iNOS inhibitor only. Based on this hypothesis, this study was designed to investigate whether melatonin or 1400W has greater protective effects on kidney damage induced by renal I/R.
MATERIALS AND METHODS
Animals and Surgery
The project was approved by the Experimental Ethics Committee of Gulhane Military Medical Academy, Ankara, Turkey, and the National Institute of Health's Guide for the Care and Use of Laboratory Animals was followed.
Thirty-two male Sprague-Dawley rats, weighing 250–300 g, were provided by the Gulhane Military Medical Academy, Experimental Research Council, and housed in standard cages at a constant temperature (24°C) and light-dark cycle in a controlled environment. Rats were fed with standard rat chow and water ad libitum.
Rats were randomly divided into four groups: sham-operated (n = 8), renal I/R (n = 8), renal I/R + melatonin (n = 8), and renal I/R + 1400W (n = 8).
Both chemicals—namely, melatonin (M5250, Sigma Chemical, St Louis, Missouri, USA) and 1400W (W4262, Sigma Chemical)—were administered at a dose of 10 mg/kg at 60 min prior to ischemia and at the beginning of reperfusion via intraperitoneal route.
Following a 12 h fasting period, animals were anesthetized with an intraperitoneal injection of ketamine hydrochloride (50 mg/kg) and xylazine (10 mg/kg). The rats were placed on a heating pad kept at 39°C to maintain constant body temperature. A midline incision was made, the renal pedicle observed and arteries bilaterally occluded with an atraumatic microvascular clamp (Bulldog Artery Clamp, Harvard Apparatus, Massachusetts, USA) for 60 min. The time of ischemia was chosen to maximize reproducibility of renal functional impairment while minimizing mortality in these animals. After 60 min of renal ischemia, clamps were removed and the kidneys were inspected for restoration of blood flow. The abdomen was closed in two layers. Sham-operated animals underwent the same surgical procedure without clamp application. Following 6 h of reperfusion period, animals were killed by cervical dislocation. At the time of death, blood was collected by heart puncture for measurement of biochemical analysis. Both kidneys were harvested for histopathological evaluation and biochemical examination.
Biochemical Analysis
Serum samples were used for the measurement of blood urea nitrogen (BUN) and serum creatinine (SCr) levels, which were used as indicators of impaired glomerular function, and aspartate aminotransferase (AST), which was used as an indicator of renal I/R injury.Citation[29] BUN, SCr, and AST were determined with an Abbott-Aeroset autoanalyzer (Chicago, Illinois, USA) using original kits.
The frozen tissues were homogenized in phosphate buffer (pH 7.4) by means of homogenization (Heidolph Diax 900; Heidolph Elektro GmbH, Kelhaim, Germany) in an ice cube. The supernatant was allocated into 2–3 separate tubes and stored at −70°C again. First, the protein content of tissue homogenates was measured by the method of Lowry et al. with bovine serum albumin as the standard. Efficacy of treatment was assessed by tissue level of malondialdehyde (MDA) using the method of Ohkawa et al., protein carbonyl content (PCC) using the method of Levine et al., superoxide dismutase (SOD) using the method of Sun et al., and glutathione peroxidase (GPx) using the method of Paglia and Valentine.[13,30,40] Nitrate and nitrite (NOx) levels, end products of nitric oxide degradation, were measured using the method described by Miranda et al., as we described in our previous works.Citation[13,30]
Histopathologic Evaluation
Both kidneys of each animal were taken for histopathologic evaluation. In all groups, samples of kidney were placed in formalin and processed through to paraffin. They were subsequently sectioned at 5 μm and stained with Hematoxylin-Eosin (H&E). The sections were scored with a previously described semiquantitative scale designed to evaluate the degree of renal damage (tubular cell necrosis, cytoplasmic vacuole formation, hemorrhage, and tubular dilatation).Citation[31] A minimum of 10 fields for each kidney slide were examined and assigned for severity of changes. The scoring system used was 0 = absent, 1 = present, and 2 = marked. Total histopathologic injury score per kidney was calculated by addition of all scores. Blind analysis of the histological samples was performed by two independent experts.
Statistical Analysis
All biochemical data are expressed as mean ± standard error of the mean (SEM). All statistical analyses were carried out using SPSS statistical software (SPSS for Windows, Version 15.0, Chicago, Illinois, USA). Differences in measured parameters among the three groups were analyzed by Kruskal-Wallis test. Dual comparisons between groups that present significant values were evaluated with Mann-Whitney U test. Statistical significance was accepted at a value of p < 0.05.
RESULTS
All animals survived throughout the experimental period.
Renal Function Markers
There was a significant increase in the SCr and BUN levels in the I/R group compared to sham-operated groups, suggesting a significant degree of glomerular dysfunction (see ). Administration of melatonin or 1400W significantly decreased the SCr and BUN levels. Renal I/R produced a significant increase in the serum AST level, used as a marker of renal injury. On the other hand, serum concentration of AST was significantly decreased in the rats administered either melatonin or 1400W. It was seen that melatonin is more efficient with renal function than 1400W based on serum biochemical markers (see ).
Table 1 Biochemical evaluation of serum for each group
Antioxidant Enzyme Activities
The renal I/R injury significantly reduced antioxidant enzyme activities (SOD and GSH-Px). Both melatonin and 1400W significantly increased antioxidant enzyme activities, but melatonin did more than 1400W (see ).
Table 2 Biochemichal evaluation of kidney for each groups
Oxidative and Nitrosative Stress Markers
The rats subjected to renal I/R revealed a strike increase in the tissue MDA and PCC levels, suggesting increased lipid peroxidation and protein oxidation. The administration of melatonin or 1400W revealed a significant decrease in the levels of MDA and PCC in the rats subjected to renal I/R (see ).
NOx level (nitrite/nitrite concentration) in the renal tissue, as as indicator of NO and peroxynitrite production, was significantly increased in the rats subjected to renal I/R. Increased tissue NOx levels were significantly decreased in the I/R + melatonin and I/R + 1400W (see ).
Histopathologic Evaluation
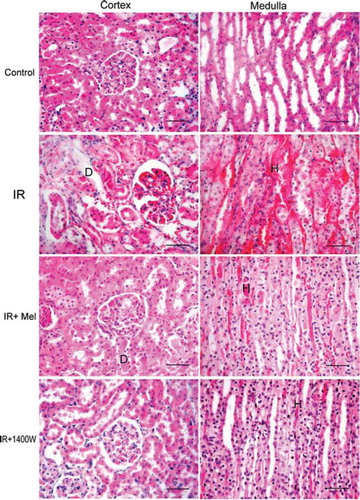
Histopathologic grading of renal injury is displayed as median (min-max) (see ). The total injury score was significantly increased in the I/R group, indicating significant renal injury. Conversely, total histological injury score was significantly decreased in the treatment groups. Although significant glomerular and tubular injury with hemorrhage and accumulation of PMNL was seen in the rats subjected to I/R, a lower degree of injury was seen in the groups treated with either melatonin and 1400W. Representative histological samples from all groups are displayed in .
Table 3 Histopathologic evaluation of kidney sections for each group
Figure 1. Representative histological photographs of kidney tissues from sham operated (control), renal ischemia reperfusion (IR) injury, renal ischemia reperfusion + melatonin (IR + Mel), and renal ischemia reperfusion + 1400W (IR + 1400W) groups. Kidney tissues were taken from cortex (right column) and medulla (left column). Upper row: Sham-operated animals show normal histological characteristic of glomeruli and tubules. Second row: Rats subjected to renal IR injury show marked necrosis with tubular dilation (D), swelling, luminal congestion, and medullar hemorrhage (H). Third row: Rats subjected to renal IR injury plus melatonin show moderate kidney damage and moderate dilatation (D) of the tubular structure. In comparison with the IR + Mel group, IR + 1400W group shows preservation of tissue histology of the kidney. (H&E, Scale bars: 50 micron).
DISCUSSION
In this study, we used melatonin as a strong antioxidant, selective iNOS inhibitor, and scavenger of ONOO−, and 1400W as a selective iNOS inhibitor, to protect kidneys against renal I/R injury in an experimental rat model. Our results clearly show that melatonin and 1400W ameliorate both oxidative and nitrosative stress in the kidneys against renal I/R injury in the rats. Interestingly, treatment with melatonin was much more effective than treatment with 1400W. The reason for this might be that melatonin is not only a strong anti-oxidants, but also an iNOS inhibitor and ONOO− scavenger.Citation[25] Apart from this, several intracellular metabolites of melatonin are well-known free radical scavengers.Citation[25]
Administration of melatonin or 1400W improved renal function, as evidenced by reduction in the levels of SCr, BUN, and AST, as compared to sham-operated animals. These findings were also confirmed with histological analyses.
In this study, we demonstrate that melatonin attenuated the degree of lipid peroxidation and protein oxidation in the kidneys of rats subjected to renal I/R injury. One of the possible explanation for this finding is that melatonin and its metabolites have the ability to scavenge many of the oxygen-based and nitrogen-based radicals, including ONOO−.Citation[25,32] In addition, when melatonin enters cellular membranes, it mainly localizes in a superficial position in lipid bilayers near the polar heads of membrane phospholipids.Citation[22,33,34] While in this position, it is obviously capable of functioning as a free radical scavenger, and it may also provide an indirect means by which the membranes resist oxidative damage. Another possible explanation is that melatonin supports several intracellular antioxidant enzymes, including SOD and GSH-Px,Citation[35,36] and induces the activity of glutamylcysteine ligase (GCL, formerly referred to as gamma-glutamylcysteine synthetase), thereby stimulating the production of another crucial intracellular antioxidant, glutathione.Citation[37] In addition, melatonin has been shown to stimulate gene expression of SOD and GSH-Px.Citation[22,33]
Regarding the ameliorative effects of 1400W, there are conflicting data for both a beneficial and a harmful effect of NO on I/R injury.Citation[38] NO is one of the most important mediators in pathophysiological changes of tissues. Under physiologic conditions, NO maintains vascular tone and inhibits aggregation and adhesion of neutrophils and platelets to vascular endothelium; these are beneficial aspects of NO function.Citation[25] Low levels of NO production protect an organ in the early stages of injury, whereas elevated and prolonged NO production by iNOS during the later stages of the insult result in or potentiate organ injury. Increased expression of iNOS was shown to contribute to I/R injury in the kidney.Citation[4,31] On the other hand, our previous work showed that inhibition of iNOS improves functional recovery in reperfused kidneys, in agreement with reports on other tissues. Taken together, the data in this study support the contention that NO produced from iNOS plays a deleterious role in kidneys subjected to renal I/R injury. The mechanism of NO-induced I/R injury may also be a result of the development of ONOO−, as NO couples with O•2−, which is also increased in response to ischemia. The production of ONOO− (nitrosative stress) occurs almost instantaneously and causes the nitration of cellular proteins with subsequent loss of protein structure and function.Citation[15,39,40] Therefore, we conclude that ONOO− is critical in renal I/R injury, and that the reduction of ONOO− may be a potential mechanism to prevent kidneys.
NO and ONOO− are eventually converted to nitrite (NO2) and/or nitrate (NO3) (i.e., NOx).Citation[21,39] Therefore, NOx levels are used as an indirect but reliable indicator for NO and ONOO− formation in vivo.Citation[39,41] In this study, we found that renal I/R caused a significant increase in tissue NOx and melatonin significantly inhibited I/R induced increases in tissue NOx. Because melatonin neutralizes ONOO−, we speculate that melatonin's protective effects were a consequence of its versatile ability to scavenge ROS and ONOO−.Citation[22,32] In addition, we believe that free oxygen radicals, NO and ONOO−, have a crucial role in injury during inflammation-induced renal I/R, and this should be kept in mind when managing or planning new studies.
In conclusion, the findings of this study demonstrated clearly that melatonin or 1400W prevents I/R injury in the kidney. NO and/or ONOO− and free oxygen radicals are involved in renal I/R injury, and the scavenging of both free oxygen radicals and peroxynitrite are crucial in reducing renal damage in ARF. Future studies may consider experimental and clinical application of melatoninCitation[42] in protecting the kidney against I/R injury.
REFERENCES
- Sheridan AM, Bonventre JV. Pathophysiology of ischemic acute renal failure. Contrib Nephrol. 2001;7–21.
- Rondon-Berrios H, Palevsky PM. Treatment of acute kidney injury: An update on the management of renal replacement therapy. Curr Opin Nephrol Hypertens. 2007;16:64–70.
- Chatterjee PK. Novel pharmacological approaches to the treatment of renal ischemia-reperfusion injury: A comprehensive review. Naunyn Schmiedebergs Arch Pharmacol. 2007;376:1–43.
- Nath KA, Norby SM. Reactive oxygen species and acute renal failure. Am J Med. 2000;109:665–678.
- Guven A, Tunc T, Topal T, Alpha-lipoic acid and ebselen prevent ischemia/reperfusion injury in the rat intestine. Surg Today. 2008;38:1029–1035.
- Mallick IH, Yang W, Winslet MC, Seifalian AM. Ischemia-reperfusion injury of the intestine and protective strategies against injury. Dig Dis Sci. 2004;49:1359–1377.
- Li C, Jackson RM. Reactive species mechanisms of cellular hypoxia-reoxygenation injury. Am J Physiol Cell Physiol. 2002;282:C227–C241.
- Greene EL, Paller MS. Oxygen free radicals in acute renal failure. Miner Electrolyte Metab. 1991;17:124–132.
- Weidig P, McMaster D, Bayraktutan U. High glucose mediates pro-oxidant and antioxidant enzyme activities in coronary endothelial cells. Diabetes Obes Metab. 2004;6:432–441.
- Cooke CL, Davidge ST. Peroxynitrite increases iNOS through NF-kappaB and decreases prostacyclin synthase in endothelial cells. Am J Physiol Cell Physiol. 2002;282:C395–C402.
- Stockklauser-Farber K, Ballhausen T, Laufer A, Rosen P. Influence of diabetes on cardiac nitric oxide synthase expression and activity. Biochim Biophys Acta. 2000;1535:10–20.
- Moncada S, Palmer RM, Higgs EA. Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142.
- Guven A, Uysal B, Akgul O, Scavenging of peroxynitrite reduces renal ischemia/reperfusion injury. Ren Fail. 2008;30:747–754.
- Korkmaz A, Topal T, Oter S, Tan DX, Reiter RJ. Hyperglycemia-related pathophysiologic mechanisms and potential beneficial actions of melatonin. Mini Rev Med Chem. 2008;8:1144–1153.
- Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: The good, the bad, and ugly. Am J Physiol. 1996;271:C1424–C1437.
- Messina A, Knight KR, Dowsing BJ, Localization of inducible nitric oxide synthase to mast cells during ischemia/reperfusion injury of skeletal muscle. Lab Invest. 2000;80:423–431.
- Takimoto Y, Aoyama T, Keyamura R, Differential expression of three types of nitric oxide synthase in both infarcted and non-infarcted left ventricles after myocardial infarction in the rat. Int J Cardiol. 2000;76:135–145.
- Iadecola C, Zhang F, Casey R, Nagayama M, Ross ME. Delayed reduction of ischemic brain injury and neurological deficits in mice lacking the inducible nitric oxide synthase gene. J Neurosci. 1997;17:9157–9164.
- Isobe M, Katsuramaki T, Kimura H, Role of inducible nitric oxide synthase on hepatic ischemia and reperfusion injury. Transplant Proc. 2000;32:1650–1652.
- Ling H, Edelstein C, Gengaro P, Attenuation of renal ischemia-reperfusion injury in inducible nitric oxide synthase knockout mice. Am J Physiol. 1999;277:F383–F390.
- Yanarates O, Guven A, Sizlan A, Ameliorative effects of proanthocyanidin on renal ischemia/reperfusion injury. Ren Fail. 2008;30:931–938.
- Reiter RJ, Tan DX. Melatonin: A novel protective agent against oxidative injury of the ischemic/reperfused heart. Cardiovasc Res. 2003;58:10–19.
- Sahna E, Parlakpinar H, Ozturk F, Cigremis Y, Acet A. The protective effects of physiological and pharmacological concentrations of melatonin on renal ischemia-reperfusion injury in rats. Urol Res. 2003;31:188–193.
- Sahna E, Parlakpinar H, Turkoz Y, Acet A. Protective effects of melatonin on myocardial ischemia/reperfusion induced infarct size and oxidative changes. Physiol Res. 2005;54:491–495.
- Tan DX, Manchester LC, Terron MP, Flores LJ, Reiter RJ. One molecule, many derivatives: A never-ending interaction of melatonin with reactive oxygen and nitrogen species?. J Pineal Res. 2007;42:28–42.
- Peyrot F, Ducrocq C. Potential role of tryptophan derivatives in stress responses characterized by the generation of reactive oxygen and nitrogen species. J Pineal Res. 2008;45:235–246.
- Mark LA, Robinson AV, Schulak JA. Inhibition of nitric oxide synthase reduces renal ischemia/reperfusion injury. J Surg Res. 2005;129:236–241.
- Ovechkin AV, Lominadze D, Sedoris KC, Inhibition of inducible nitric oxide synthase attenuates platelet adhesion in subpleural arterioles caused by lung ischemia-reperfusion in rabbits. J Appl Physiol. 2005;99:2423–2432.
- Chatterjee PK, Patel NS, Kvale EO, Inhibition of inducible nitric oxide synthase reduces renal ischemia/reperfusion injury. Kidney Int. 2002;61:862–871.
- Yanarates O, Guven A, Sizlan A, Ameliorative effects of proanthocyanidin on renal ischemia/reperfusion injury. Ren Fail. 2008;30:931–938.
- Rabb H, Ramirez G, Saba SR, Renal ischemic-reperfusion injury in L-selectin-deficient mice. Am J Physiol. 1996;271:F408–F413.
- Reiter RJ, Tan DX, Jou MJ, Biogenic amines in the reduction of oxidative stress: Melatonin and its metabolites. Neuro Endocrinol Lett. 2008;29:391–398.
- Reiter RJ, Tan DX, Osuna C, Gitto E. Actions of melatonin in the reduction of oxidative stress. A review. J Biomed Sci. 2000;7:444–458.
- Ceraulo L, Ferrugia M, Tesoriere L, Interactions of melatonin with membrane models: Portioning of melatonin in AOT and lecithin reversed micelles. J Pineal Res. 1999;26:108–112.
- Rodriguez C, Mayo JC, Sainz RM, Regulation of antioxidant enzymes: A significant role for melatonin. J Pineal Res. 2004;36:1–9.
- Reiter RJ, Tan DX, Maldonado MD. Melatonin as an antioxidant: Physiology versus pharmacology. J Pineal Res. 2005;39:215–216.
- Winiarska K, Fraczyk T, Malinska D, Drozak J, Bryla J. Melatonin attenuates diabetes-induced oxidative stress in rabbits. J Pineal Res. 2006;40:168–176.
- Lin WY, Levin RM, Chichester P, Effects of L‐arginine and L-NAME on chronic partial bladder outlet obstruction in rabbit. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2390–R2399.
- Tunc T, Uysal B, Atabek C, Erdosteine and ebselen as useful agents in intestinal ischemia/reperfusion injury. J Surg Res. 2008;155(2):210–216.
- Oztas E, Guven A, Turk E, 3-aminobenzamide, a poly ADP ribose polymerase inhibitor, attenuates renal ischemia/reperfusion injury. Ren Fail. 2009;31(5):393–399.
- Guven A, Gundogdu G, Uysal B, Hyperbaric oxygen therapy reduces the severity of necrotizing enterocolitis in a neonatal rat model. J Pediatr Surg. 2009;44:534–540.
- Korkmaz A, Reiter RJ, Topal T, Melatonin: An established antioxidant worthy of use in clinical trials. Mol Med. 2009;15:43–50.
