Abstract
Acute renal failure (ARF) is common after cardiac surgery and more frequent after complex cardiac surgery. While the incidence of ARF is increasing after coronary artery bypass graft (CABG) surgery, trends in other forms of cardiac surgery remain unclear. We investigated the trend of ARF in various cardiac procedures and compared patterns using CABG surgery as a reference group. The study population consisted of discharges from the Nationwide Inpatient Sample from 1988 to 2003, grouped according to surgery as: CABG, CABG with mitral valve, CABG with other valve, valve alone, and heart transplant. Standard diagnostic codes were used to identify ARF among discharges. Multivariable regression was used to determine trends in ARF among various procedures with CABG as a reference group. The incidence of ARF increased in all five groups (p < 0.001) over the 16-year period. The ARF incidence was highest in the heart transplant group (17%). Compared to the CABG population, patients following heart transplantation developed ARF at higher rates during the study period. In contrast, while ARF increased over time in other groups, the rates of rise were slower than in CABG patients. Among heart surgery procedures, ARF incidence is highest in heart transplantation. The incidence of ARF is also increasing at a faster rate in this group of patients in contrast to other procedure groups when compared to CABG surgery. The disproportionate increase in ARF burden after heart transplantation is a concern due to its strong association with chronic kidney disease and mortality.
INTRODUCTION
Acute renal failure (ARF) is a major postoperative morbidity that complicates 1–8% of cardiac surgeries.Citation[1–4] Postoperative ARF increases resource utilization, hospital length of stay, total health care costs, and risk of both short- and long-term mortality.Citation[4–7] Acute renal failure has been noted to occur in up to 9% of heart transplant recipientsCitation[8–10] and in 7.5% of patients following valve surgery.Citation[1,3] This suggests that the incidence of ARF is variable among different cardiac surgical procedures, with heart transplant recipients at higher risk. The occurrence of ARF after heart transplantation is significant due to the strong association with progression to chronic kidney disease, increased resource utilization, and mortality.Citation[11,12]
Various criteria have been used to define ARF, including absolute and relative increase in creatinine and the need for renal replacement therapy,Citation[13,14] making comparisons between studies difficult. While ARF may be regarded as a clinical diagnosis, acute kidney injury has been proposed as the appropriate term that defines an acute renal insult in any setting, and is the preferred research definition. However, despite variable definitions, ARF remains the standard diagnosis in clinical practice and is still uniformly included in research and administrative databases. Using national databases that include ARF as a clinical diagnosis, studies have reported an increasing incidence of ARF among all hospitalized patients.Citation[15] Recently, we reported a rising trend in ARF following coronary artery bypass graft (CABG) surgery in the United States.Citation[16] However, while the epidemiology of ARF following CABG surgery is being extensively studied,Citation[17] ARF trends in other forms of cardiac surgery remain unclear. Therefore, using a large national inpatient database, we investigated the trend in ARF in various cardiac procedures including heart transplantation. In addition, we compared ARF trends among cardiac surgical procedures to determine whether specific procedures remain at higher risk compared to isolated CABG surgery.
MATERIALS AND METHODS
Data Sources
We used data from the Nationwide Inpatient Sample (NIS) database to investigate the trend in ARF in different cardiac surgery procedures. The NIS is the largest database available for tracking information on inpatients in the United States and contains information from more than 1000 hospitals in 37 states. This represents a 20% stratified sample of U.S. community hospitals, correlating with 90% of hospital discharges in this country. The NIS database was developed by the Agency for Healthcare Research and Quality in order to provide a large sample size to study trends, rare conditions, and uncommon treatments. This database does not contain any patient identifiers and was approved by the Duke University Institutional Review Board as exempt from review. All members of the research team with access to the NIS database have a Data Use Agreement on file. All available discharge data over a 16-year period (1988–2003) from the NIS database were used in this study.
Identification of Study Population
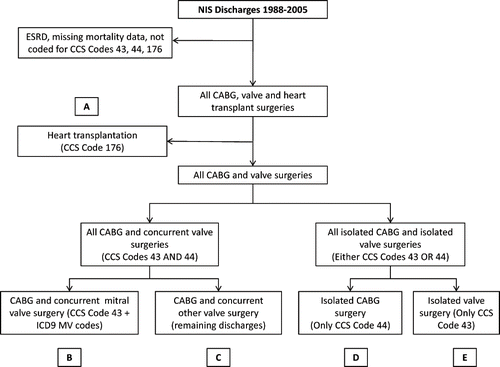
The study population consisted of discharges containing clinical classification software (CCS) codes for CABG (code 44), valve surgery (code 43), or heart transplantation (code 176), as shown in . All discharges coded for end stage renal disease and missing mortality data were excluded.
Table 1 Procedural codes used in this study
We defined our study groups in a hierarchical manner as shown in . First, discharges with the CCS code for heart transplantation were identified (Group A). Next, the remaining discharges were dichotomized according to the presence of either both CCS codes for CABG and valve surgery or only one of these codes. Those with both procedure codes represented the CABG with combined valve surgery population, while those with only one of these codes comprised the isolated CABG or valve procedure. Within the combined procedure population, those with mitral valve surgery procedure codes (including International Classification of Disease 9th revision Clinical Modification [ICD9-CM] codes [3502, 3512, 3523, and 3524]) comprised Group B (CABG and mitral valve), while Group C included the remaining CABG and “other” valve surgery population. Within the isolated CABG or valve procedure population, those with only the CABG procedure code comprised Group D (isolated CABG), while those with only a valve procedure code represented group E (isolated valve).
Figure 1. Hierarchical methodology of definition of groups based on surgical procedure (see text for details). Abbreviations: CABG = coronary artery bypass graft, CCS = clinical classification software, ESRD = end stage renal disease, ICD9-CM = International Classification of Disease 9th Revision Clinical Modification, MV = mitral valve, NIS = Nationwide Inpatient Sample.
Statistical Analyses
After the five groups were established, we determined the incidence of ARF in each group. This was done using the ICD9-CM diagnosis codes for ARF (584.0, 584.5–584.9, and 586; see ). Demographic and co-morbidity information was available for all discharges. The analyses were performed using age, female gender, number of valves repaired, number of bypass grafts performed, and a comprehensive set of 29 comorbidities described by Elixhauser.Citation[18] Comorbidities included diabetes, hypertension, chronic obstructive pulmonary disease, obesity, peripheral vascular disease, neurological disorders, anemia, coagulopathy, and other less common conditions. Valvular heart disease was not included as a co-morbidity, as this disease process was accounted for by the surgical groups.
Estimations of incidence rates were calculated using the year of surgery as a categorical variable. A separate multivariable analysis was performed using the year of surgery as a continuous variable in order to estimate the slope of ARF rates over time. Additionally, an overall multivariable model was constructed using all five surgical procedures, with a group-year interaction variable included in the model for the purpose of comparing slopes over time. Because logistic regression modeling produces a sigmoid curve of predicted probabilities, the logit function was used to transform probabilities (inverse of sigmoid function) and produce straight lines, simplifying comparisons among groups. In this model, each procedure group was compared using isolated CABG surgery (group D) as a reference to determine if the incidence of ARF among the different procedures was increasing at the same rate as isolated CABG surgery. The ARF slope for Group D was therefore given a value of 0. Consequently, a positive slope indicated that the rate of increase in ARF was higher than that of Group D, whereas a negative slope indicated the rate of increase was slower than that of the reference group.
Discharge weights were applied to all analyses to obtain national estimates. Significance was assessed at an alpha level of 0.05. All analyses were conducted using SAS 9.1 statistical software (SAS Inc., Cary, North Carolina, USA).
RESULTS
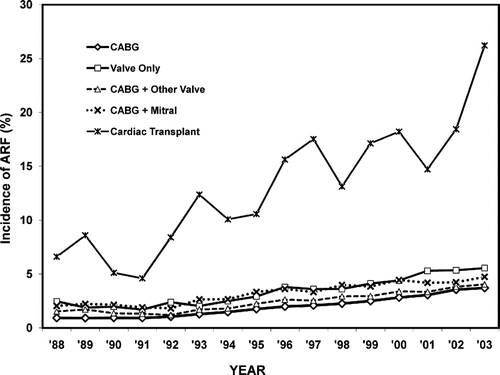
During the study period (1988–2003), an estimated 8,990,620 cardiac surgeries were performed in the United States. Group-wise distribution of the study population is shown in . A description of demographics, procedural variables, co-morbidities, and frequency of ARF is also provided in this table. The cumulative incidence of ARF over the study time period in the heart transplant group was 17%, which was higher than the other groups (range: 3.6 to 11.8%).
Table 2 Group-wise distribution of the study population
The primary multivariable logistic regression analysis modeling ARF incidence showed that year was significantly associated with ARF for each group (p < 0.001). The model also accounted for age, female gender, and 29 other comorbidities as described previously. The time trend of the adjusted incidence of ARF over the study period is shown in . The largest increase in incidence of ARF was observed in Group A (heart transplantation: from 6.6% in 1988 to 26.2% in 2003).
Figure 2. The incidence of ARF in each group (A-E) from 1988 to 2003. Abbreviations: ARF = acute renal failure, CABG = coronary artery bypass graft.
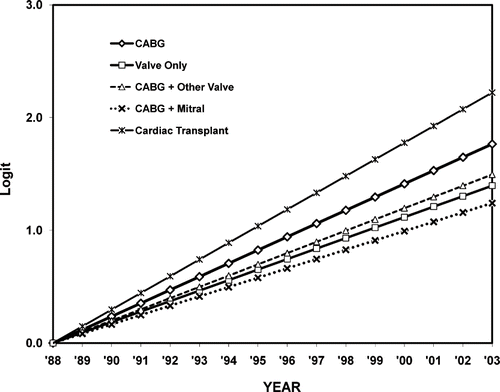
The secondary multivariable regression analysis of ARF rates among different groups showed that slopes in all comparison groups were significantly different (p < 0.001) from the reference group (see ). While the incidence of ARF was increasing at a lower rate in groups B, C and E, the rate of rise in ARF was significantly higher than the reference group in only the heart transplantation cohort (p < 0.0001). The logits from this model are shown in . Intercepts were set to a value of 0 in 1988 to simplify the comparison of slopes among groups.
Table 3 Multivariable regression model comparing slopes
Figure 3. A multivariable regression model comparing the rates of ARF among all groups to the reference CABG only group. The heart transplant group had a positive slope, indicating that the incidence of ARF in this group was rising faster than that in the CABG-only group. All other groups had negative slopes (see text for details). Abbreviations: ARF = acute renal failure, CABG = coronary artery bypass graft.
DISCUSSION
Our study highlights two major issues in the epidemiology of ARF associated with cardiac surgery. First, the incidence of ARF in the heart transplantation population is significantly higher than other forms of cardiac surgery; and second, the rate of rise in ARF in the transplant group has been disproportionately higher than any other cardiac surgery procedure. In contrast, the rate of rise in ARF in isolated valve procedures and combined CABG-valve surgery was slower that the isolated CABG surgery group.
The epidemiology of ARF has been studied in a number of settings. Waiker et al. showed a rising trend in ARF among hospitalized patients,Citation[15] and our group reported similar findings in the CABG population.Citation[16] ARF has also been well characterized in terms of its incidence, risk factors, and etiology by several investigators.Citation[2,4,17,19] However, most epidemiologic studies of postoperative ARF have been limited to the CABG or valve surgery population. Studies suggest a high risk of ARF after heart transplantation.Citation[9,20] Reasons attributed to this risk range from a heightened inflammatory response related to the procedure, pre-existing renal insufficiency from heart failure, and nephrotoxic effects of immunosuppression.Citation[20–22] The need for immunosuppression following transplantation, particularly cyclosporine, may contribute to the high incidence of ARF after heart transplantation. The NIS database does not contain immunosuppression data and began data collection only in 1988. As cyclosporine was introduced in the early 1980s, it is difficult to ascertain if its use had any influence on our observations on ARF incidence. Perhaps with the development and use of newer agents, the alarming trend of ARF we observed in this population will improve over time. While the higher risk of ARF after heart transplantation is already known, our study highlights the increasing incidence over time that is disproportionate with other forms of cardiac surgery. The risk of ARF associated with heart transplantation is higher now and seems to be increasing.
There may also be other factors influencing the increasing incidence of ARF in heart transplant recipients. Boyle et al. determined several preoperative risk factors for ARF (defined as the need for postoperative hemodialysis) in this patient population, including previous cardiac surgery.Citation[8] The recent increase in the use of mechanical devices for circulatory support as a bridge to transplant has resulted in more patients presenting for heart transplantation who have had prior cardiac surgery. This may partly explain the increasing rate of ARF that we observed in this patient population.
The use of aprotinin may contribute to a higher incidence of ARF after cardiac surgery.Citation[23–25] While the increase in the use of this popular antifibrinolytic in later years may be a factor in our observations, the lack of detailed medication data in the NIS precludes an investigation of this phenomenon.
The occurrence of postoperative ARF has serious implications for the heart transplant population. It has been associated with the development of long-term chronic renal failureCitation[11] and with a high risk of mortality.Citation[8,9] Our study shows not only a substantial incidence of ARF in heart transplant surgery, but also a disturbing rate of increase in incidence. It is therefore necessary to focus our efforts into establishing the etiology of ARF in heart transplant recipients and to determine mechanisms of prevention and renal salvage.
Our study also found that while the incidence of ARF in patients undergoing valvular procedures and combined CABG and valve surgery is increasing, the rate of rise is slower than those undergoing CABG alone. This was an unexpected finding, as valve surgery has been shown to be an independent risk factor for ARF by Grayson et al., and combined CABG-valve surgery is associated with a higher risk of postoperative ARF than CABG alone.Citation[3] Alternatively, minimally invasive valve surgery, which is increasing in popularity, has been shown to be associated with a decreased incidence of ARF compared to those patients who undergo a median sternotomy.Citation[26,27] Improvements in surgical techniques over time and earlier intervention prior to the development of heart failure may partly explain our observation of a slower rate of increase in ARF in this setting. Another factor may be that CABG surgery is being increasingly performed in patients with complex coronary artery disease not amenable to percutaneous intervention compared with previous years. This complexity may affect outcomes in two ways. First, complex coronary artery disease may translate to longer revascularization times, which may influence the occurrence and severity of postoperative ARF. Second, isolated CABG patients may be getting older and with higher number of comorbidities, therefore predisposing them to a higher risk of adverse outcomes. However, our analyses included both the number of bypass grafts performed (as a surrogate marker of procedure duration) and age and comorbid conditions, and the observed trend persisted despite accounting for these variables.
The principal strength of our study is a large sample size as well as representation from a wide geographical area across the United States over a long timeframe. However, the fact that the NIS is an administrative database presents some limitations. Administrative databases typically lack detailed data found in research databases, are dependent on coding accuracy, and lack laboratory and follow-up data precluding further investigation of observed phenomena. For instance, the true incidence of acute renal function impairment cannot be investigated in terms of acute kidney injury, as creatinine data are unavailable. Therefore the assessment of acute kidney injury is dependent on the clinical diagnosis of ARF, regardless of definition, and coding accuracy. Lorence et al. found a 5–20% discrepancy between coders and physicians.Citation[28] Coding inaccuracy has also been shown to be dependent on institution size, location, and academic status.Citation[28,29] However, ARF is more likely to be coded as a discharge diagnosis when present than when absent. Two groups have attempted to validate the accuracy of ICD-9-CM codes for ARF.Citation[30,31] These investigators found that although codes for ARF and chronic renal failure were more specific than sensitive, they were sufficiently robust for evaluating trends, especially when associated with a dialysis procedure. The NIS database was developed by the Agency for Healthcare Research and Quality with the specific purpose of assessing trends in less common diseases that would otherwise be difficult with smaller datasets or require multi-institutional collaborative initiatives. The NIS data are also subject to periodic review and quality control of the information it contains, making it a reliable and useful tool to evaluate trends over time.
In summary, using a nationwide database, we found that the incidence of ARF was not only high among heart transplant recipients, but was increasing at a disproportionate rate in this population compared with other forms of cardiac surgery. Increase in heart transplantation in patients with history of prior cardiac surgery and use of potentially nephrotoxic immunosuppression may partly explain our findings. An unexpected slower rate of increase in ARF in combined CABG and valve surgery may reflect improved surgical management and/or earlier intervention. The increasing rate of ARF in heart transplantation is a concern given the high risk of progression to chronic kidney disease and long-term mortality associated with this complication. Our findings suggest that further investigation into mechanisms of ARF and assessment of protective interventions is warranted, especially in the heart transplant population. The risk of ARF may also be considered in discussions with patients and their families to help with decision-making in the perioperative period.
DECLARATION OF INTEREST
The authors report no conflicts of interest.
REFERENCES
- Chan V, Jamieson WR, Chan F, Germann E. Valve replacement surgery complicated by acute renal failure—predictors of early mortality. J Card Surg. 2006;21:139–143.
- Chertow GM, Lazarus JM, Christiansen CL, Preoperative renal risk stratification. Circulation. 1997;95:878–884.
- Grayson AD, Khater M, Jackson M, Fox MA. Valvular heart operation is an independent risk factor for acute renal failure. Ann Thorac Surg. 2003;75:1829–1835.
- Mangano CM, Diamondstone LS, Ramsay JG, Aggarwal A, Herskowitz A, Mangano DT. Renal dysfunction after myocardial revascularization: Risk factors, adverse outcomes, and hospital resource utilization. The Multicenter Study of Perioperative Ischemia Research Group. Ann Intern Med. 1998;128:194–203.
- Thakar CV, Worley S, Arrigain S, Yared JP, Paganini EP. Influence of renal dysfunction on mortality after cardiac surgery: Modifying effect of preoperative renal function. Kidney Int. 2005;67:1112–1119.
- Brown JR, Cochran RP, Dacey LJ, Perioperative increases in serum creatinine are predictive of increased 90‐day mortality after coronary artery bypass graft surgery. Circulation. 2006;114:I409–I413.
- Chertow GM, Levy EM, Hammermeister KE, Grover F, Daley J. Independent association between acute renal failure and mortality following cardiac surgery. Am J Med. 1998;104:343–348.
- Boyle JM, Moualla S, Arrigain S, Risks and outcomes of acute kidney injury requiring dialysis after cardiac transplantation. Am J Kidney Dis. 2006;48:787–796.
- Canver CC, Heisey DM, Nichols RD. Acute renal failure requiring hemodialysis immediately after heart transplantation portends a poor outcome. J Cardiovasc Surg (Torino). 2000;41:203–206.
- Odim J, Wheat J, Laks H, Peri-operative renal function and outcome after orthotopic heart transplantation. J Heart Lung Transplant. 2006;25:162–166.
- Ojo AO, Held PJ, Port FK, Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003;349:931–940.
- Hornberger J, Best J, Geppert J, McClellan M. Risks and costs of end-stage renal disease after heart transplantation. Transplantation. 1998;66:1763–1770.
- Stafford-Smith M, Patel UD, Phillips-Bute BG, Shaw AD, Swaminathan M. Acute kidney injury and chronic kidney disease after cardiac surgery. Adv Chronic Kidney Dis. 2008;15:257–277.
- Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–R212.
- Waikar SS, Curhan GC, Wald R, McCarthy EP, Chertow GM. Declining mortality in patients with acute renal failure, 1988 to 2002. J Am Soc Nephrol. 2006;17:1143–1150.
- Swaminathan M, Shaw AD, Phillips-Bute BG, Trends in acute renal failure associated with coronary artery bypass graft surgery in the United States. Crit Care Med. 2007;35:2286–2291.
- Cooper WA, O'Brien SM, Thourani VH, Impact of renal dysfunction on outcomes of coronary artery bypass surgery: Results from the Society of Thoracic Surgeons National Adult Cardiac Database. Circulation. 2006;113:1063–1070.
- Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27.
- Conlon PJ, Stafford-Smith M, White WD, Acute renal failure following cardiac surgery. Nephrol Dial Transplant. 1999;14:1158–1162.
- Cruz DN, Perazella MA. Acute renal failure after cardiac transplantation: A case report and review of the literature. Yale J Biol Med. 1996;69:461–468.
- Greenberg A, Thompson ME, Griffith BJ, Cyclosporine nephrotoxicity in cardiac allograft patients—a seven‐year follow-up. Transplantation. 1990;50:589–593.
- Myers BD, Newton L. Cyclosporine-induced chronic nephropathy: An obliterative microvascular renal injury. J Am Soc Nephrol. 1991;2:S45–S52.
- Shaw AD, Stafford-Smith M, White WD, The effect of aprotinin on outcome after coronary-artery bypass grafting. N Engl J Med. 2008;358:784–793.
- Mangano DT, Tudor IC, Dietzel C. The risk associated with aprotinin in cardiac surgery. N Engl J Med. 2006;354:353–365.
- Karkouti K, Beattie WS, Dattilo KM, A propensity score case-control comparison of aprotinin and tranexamic acid in high-transfusion-risk cardiac surgery. Transfusion. 2006;46:327–338.
- McCreath BJ, Swaminathan M, Booth JV, Mitral valve surgery and acute renal injury: Port access versus median sternotomy. Ann Thorac Surg. 2003;75:812–819.
- Antonic M, Gersak B. Renal function after port access and median sternotomy mitral valve surgery. Heart Surg Forum. 2007;10:E401–E407.
- Lorence DP, Ibrahim IA. Disparity in coding concordance: Do physicians and coders agree?. J Health Care Finance. 2003;29:43–53.
- Rangachari P. Coding for quality measurement: The relationship between hospital structural characteristics and coding accuracy from the perspective of quality measurement. Perspect Health Inf Manag. 2007;4:3.
- Waikar SS, Wald R, Chertow GM, Validity of International Classification of Diseases, ninth revision, clinical modification codes for acute renal failure. J Am Soc Nephrol. 2006;17:1688–1694.
- Quan H, Parsons GA, Ghali WA. Validity of information on comorbidity derived from ICD-9-CCM administrative data. Med Care. 2002;40:675–685.
