Abstract
Background. To evaluate the effects of everolimus on renal ischemia-reperfusion injury (IRI). Methods. Wistar albino rats were divided into control, ischemia-reperfusion (IR), and ischemia-reperfusion/everolimus (IR/eve) groups. Everolimus was administered for seven consecutive days to the IR/eve group prior to injury. IR and IR/eve groups underwent forty-five minutes ischemia followed by the application of reperfusion at 2 and 24 hours. Blood samples and kidneys were taken from all animals. Results. Serum blood urea nitrogen and creatinine levels increased at two hours of reperfusion in the IR and IR/eve groups, and decreased at 24 hours of reperfusion in the IR group. In the IR/eve group, we detected significantly high interleukin-6 levels and low tumor necrosis factor-α and malondialdehyde levels at 24 hours. Myeloperoxidase levels increased at two hours of reperfusion in the IR/eve group, but decreased significantly at 24 hours. Everolimus did not improve renal tubular and interstitial injuries in renal IRI. Conclusions. It has been demonstrated that pretreatment with everolimus has beneficial effects on cytokines and oxidative stress in renal IRI. However, these effects are insufficient for the correction of histopathological changes and restoration of normal kidney function.
INTRODUCTION
Ischemia-reperfusion injury (IRI) to the kidney is initiated by an abrupt decrease in renal blood flow due to prerenal causes and vasoconstriction or obstruction in intrarenal microvasculature.Citation[1–3] Although mechanisms underlying the renal IRI are not understood fully, evidence from previous research suggests that there is an inflammatory response involving endothelial injury, enhanced leucocyte-endothelial interactions by means of increased expression of adhesion molecules, and increased production of cytokines.Citation[1,3,4]
A macrolide immunosuppressant used in renal transplantation, everolimus, inhibits the proliferation of both hematopoietic and vascular smooth muscle cells.Citation[5–7] In vitro studies have shown that its application decreases the release of TNF-α and TNF-α-induced IL-6.Citation[8] Cellular protective and anti-inflammatory effects have been studied in the treatment of ischemia-reperfusion (IR) with sirolimus, from which everolimus is derived. Sirolimus has the same action as everolimus and was observed to decrease TNF-αCitation[8,9] and TNF-α-induced IL-6 levels.Citation[8] Sirolimus was also shown to have an encouraging role on total antioxidant stress status and malondialdehyde levels (MDA).Citation[10]
The present study was designed to evaluate the effects of everolimus on renal IRI. Given that cytokines and T and B cells play a role in the pathogenesis of renal IRI, we hypothesized that pretreatment with everolimus would improve renal functions and renal histology through the inhibition of T cell proliferation and reduction in levels of TNF-α and IL-6. Our study investigated the effects of prophylactic everolimus usage on renal functions, lipid peroxidation, cytokines, antioxidant systems, and renal histology.
METHODS
Animals
The study sample comprised Wistar albino rats weighing between 200–250g and maintained on a standard diet with free access to tap water.
Experimental Design and Operative Technique
We divided the study sample into three groups: control, ischemia-reperfusion (IR), and ischemia-reperfusion/everolimus (IR/eve).
Everolimus Administration
Everolimus provided by Novartis was dissolved in water and given by gastric gavage prior to the surgical procedure. Because the everolimus reached steady state in seven days, the everolimus at a dosage of 1.5 mg/kg, which was determined according to the literature, was given for seven consecutive days before the surgical procedure.Citation[11,12] The IR group was given only saline by gastric gavage.
The rats were anesthetized with an injection of 40 mg/kg ketamine i.m. The abdomen was shaved and a midline incision was performed. Blood samples and kidneys of the control group were taken to determine normal values. No other surgical procedure was applied to the control group (n = 10). The renal pedicles of the rats in the IR and IR/eve groups were cross-clamped for 45 minutes. Two hours after reestablishment of the blood flow, blood samples and kidneys were taken from 11 subjects in the IR group and from 9 in the IR/eve group. The same procedure was undertaken at 24 hours of reperfusion for seven animals from the IR group and nine from the IR/eve group.
Renal Function, TNF-α, and IL-6 Assessment
Serum blood urea nitrogen (BUN), creatinine (Cr), TNF-α, and IL-6 levels were assessed in the control group and the IR and IR/eve groups at the end of reperfusion periods (at 2 and 24 hours of reperfusion). Serum BUN and Cr levels were used as indicators of renal functions and measured by an automatic analyzer (Beckman Coulter LX20). Serum TNF-α and IL-6 levels were obtained by using enzyme linked immunosorbent (ELISA) kits (Biosource International, USA) in accordance with the manufacturer's instructions.
Malondialdehyde (MDA) Assay
The effect of IR on lipid peroxidation was evaluated by measuring MDA levels in kidney tissue as previously described by Ohkawa.Citation[13]
Superoxide Dismutase (SOD) Assay
As superoxide dismutase is an important enzyme in the neutralization of free oxygen radicals in IRI, we established the SOD activities of the rats using the method described by Yi-Sun for kidneys.Citation[14]
Myeloperoxidase (MPO) Assay
MPO activity was measured as an indicator of neutrophil and mononuclear cell infiltration as previously described.Citation[15]
Renal Histopathology
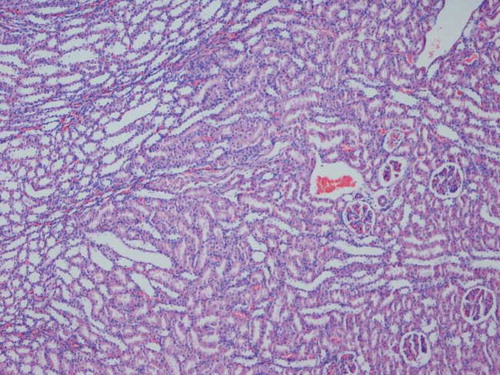
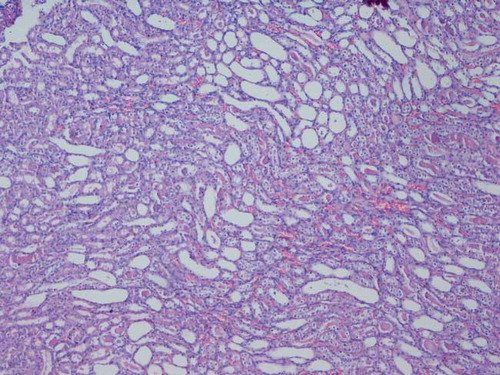
Harvested kidneys were fixed in 10% phosphate buffered formalin. Paraffin embedded specimens were cut into 6 μm-thick sections and stained with hematoxylin-eosin. Samples were examined for tubular dilatation, inflammatory cell infiltration, cast formation in the tubular lumen, and tubular epithelial cell detachment, and they were scored as normal, mild, moderate and severe according to the level of injury.Citation[16]
The study protocol was approved by the institute's committee on animal research and conformed to institutional standards for experiments on animals.
Statistics
Data are expressed as means ±standard deviation. They were compared using the Mann-Whitney U test (results did not show normal distribution) with software package SPSS. Categorized histopathological results were compared by using the Fischer Exact test. Statistical significance was determined as p < 0.05.
RESULTS
All results are displayed in .
Table 1 Biochemical results in our study
Renal Functions
In our study, BUN levels increased significantly more at two hours of reperfusion in both the IR and IR/eve groups in comparison to the control group (p < 0.05). Blood urea nitrogen levels in the IR group were improved at 24 hours of reperfusion and decreased below the levels of BUN in the controls. The same improvement was not seen in the IR/eve group, where BUN levels continued to be higher at 24 hours of reperfusion.
Creatinine concentrations in both the IR and IR/eve groups demonstrated significant elevations at two hours of reperfusion compared to the control group (p < 0.05). Although creatinine levels at 24 hours of reperfusion showed a reduction in both groups, this reduction was not significant in the IR/eve group, while creatinine levels decreased to near normal levels in the IR group compared to control group.
TNF-α
Everolimus pretreatment was observed to reduce TNF-α levels at both 2 and 24 hours of reperfusion, but these were not significant in comparison to the control group. However the decrease in TNF-α levels was statistically significant when compared to the IR group at two hours of reperfusion (p < 0.05). In the IR group, TNF-α levels increased significantly at two hours of reperfusion compared to the control group and decreased significantly at 24 hours of reperfusion (p < 0.05).
IL-6
When compared to the control group, IL-6 levels in the IR group increased at two hours of reperfusion and decreased at 24 hours. In the IR/eve group, IL-6 levels increased significantly at two hours of reperfusion compared to the control group, and this increase was also observed at 24 hours. When compared to the IR group, the IL-6 levels of the IR/eve group were increased at two hours of reperfusion and significantly increased at 24 hours.
MPO
Myeloperoxidase levels in the IR group demonstrated elevation at both two and 24 hours of reperfusion, which was significant at two hours of reperfusion when compared to the control group (p < 0.05). In the IR/eve group, MPO levels did not show a significant elevation at two hours of reperfusion and even showed a decrease at 24 hours when compared to the control group. MPO levels in the IR/eve group were lower than the IR group at two and 24 hours of reperfusion, and the decrease was significant at 24 hours.
MDA
When compared to the control group, MDA levels in the IR group increased at both 2 and 24 hours of reperfusion, which was significant at 24 hours (p < 0.05). Everolimus pretreatment registered a significant improvement in MDA levels in at both 2 and 24 hours of reperfusion (p < 0.05). When compared with the control group, the IR/eve group showed a decrease in MDA levels at both 2 and 24 hours of reperfusion.
SOD
In the IR group, SOD levels decreased at 2 and 24 hours of reperfusion and the decrease was significant at two hours in comparison to the control group (p < 0.05). SOD levels in the IR/eve group showed significant reduction at both 2 and 24 hours of reperfusion compared to the control group (p < 0.05). No significant difference was observed between SOD levels in the IR and IR/eve groups.
Histopathological Changes
Histological examination of kidneys obtained from the IR and IR/eve groups revealed similar tubular dilatation, detachment of tubular epithelium, cast formation, and inflammatory cell infiltration (see and ).
DISCUSSION
In this study, we considered the effects of everolimus as a protective agent in the renal IR injury (IRI) model and examined renal functions, lipid peroxidation, cytokines, inflammation, and antioxidant systems.
Tumor necrosis factor-α is a proinflammatory cytokine secreted from macrophages, leukocytes, and renal tubular cells during renal IRI.Citation[18] Although 30 minutes is considered sufficient time for renal TNF-α mRNA production, the expression and bioactivity of TNF-α protein peaks at one hour of ischemia followed by two hours of reperfusion.Citation[20,21] It is known that inhibition of TNF activity with TNF binding protein prolongs renal allograft survival in nonhuman primates.Citation[18,19] It has been shown that treatment with sirolimus, from which everolimus is derived and which has the same mechanism of action, inhibits TNF-α release from vascular smooth muscle cells.Citation[17] Everolimus was also found to reduce the release of TNF-α and TNF-α induced IL-6Citation[8] during in vitro studies. In our study, we aimed to decrease the TNF-α production leading to the prevention of renal injury by using everolimus in the treatment. In concordance with this, TNF-α levels in the IR/eve group decreased significantly at two hours of reperfusion compared to the IR group. The results of our study, which demonstrates the inhibition effect of everolimus on TNF-α release, are consistent with the literature concerning everolimus and sirolimus.Citation[8,9,17]
Interleukin-6 is a pleiotropic cytokine known to affect the regulation of immunological and inflammatory response. Some uncertainty exists about its role in tissue injury and inflammation associated with IRI. In a recent study using IL-6 knockout mice and mice that had been administered IL-6 antibodies, the expression of ICAM-1 and P selectin and production of TNF-α and IL-1β were observed to decrease, leading to protection from renal IRI.Citation[22] In contrast, another study on mice demonstrated that statins protected from renal IRI and IL-6 levels were increased. It was suggested that elevated levels of IL-6 prevented IRI.Citation[23] Similarly, in our study, IL-6 levels in the IR/eve group were found to increase when compared to both the control and IR groups, while TNF-α levels decreased with everolimus treatment. The role of TNF-α and MDA in IRI is definite, so it should be convenient to assess the effects of IL-6 in IRI on the basis of TNF-α and MDA levels in our study. Because TNF-α and MDA levels, which are important indicators of ischemic injury, were observed to decrease in the IR/eve group in our study, the increase of IL-6 in IR/eve seems to be protective against renal IRI.
Free oxygen radicals produced during the reperfusion phase of renal IRI cause cellular destruction by means of lipid peroxidation. While SOD is a biological antioxidant that plays a role in attenuating the injury caused by free oxygen radicals, MDA is an end-product of lipid peroxidation and gives information about tissue damageCitation[24,25] in IRI. In our study, tissue MDA levels of the IR/eve group decreased significantly at both 2 and 24 hours of reperfusion compared to both control and IR groups. This finding suggests that everolimus may be protective against renal IRI by decreasing lipid peroxidation. Accordingly, SOD activity was expected to be higher in the IR/eve group; however it exhibited a reduction in both the IR and IR/eve groups. This may be explained by IR models in which the period of ischemia was found to affect SOD activities. While short-term ischemia (30 minutes) does not affect SOD activity, long-term ischemia (60 or 90 min) results in a decrease in activity. It is known that there is a direct correlation between SOD activity and the period of ischemia applied to the kidney.Citation[26] In our study, 45 min of ischemia was applied to the rats in both the IR and IR/eve groups, which may have contributed to the decrease in SOD activity observed in both groups.
We also investigated MPO levels, which indicate both neutrophil and mononuclear cell infiltration to the injured tissue and are elevated soon after the ischemic injury.Citation[27] Myeloperoxidase levels in the IR group increased significantly compared to the control group, as expected. When compared with the IR and control groups, MPO levels in the IR/eve group decreased, indicating that everolimus prevents neutrophil and mononuclear cell infiltration to the ischemic tissue. As a result, everolimus was seen to produce an anti-inflammatory in addition to an inhibitory effect on T and B cell proliferation.
In our study, BUN and Cr levels increased in the IR/eve group in comparison to both the control and IR groups at 2 and 24 hours of reperfusion even though this difference was not significant statistically. This indicates that BUN and Cr levels do not confer the beneficial effects of everolimus on MDA, MPO, TNF-α, and IL-6 levels, although such beneficial effects would be expected to be protective against renal IRI. In the literature it was reported that everolimus can cause glomerulosclerosis as a toxic effect when given at high doses.Citation[28] Nevertheless, no glomerulosclerosis was observed during the histopathological examination. While there is not yet clear evidence about the nephrotoxicity of everolimus, there have been recent data about sirolimus where, in a renal IR model, it was observed to impair renal functions and tubular regeneration.Citation[29] Consistent with this data, in a renal artery occlusion model, recovery of renal functions was delayed in sirolimus-treated animals. The same study found that the increased apoptosis of tubular cells was probably mediated by inhibition of p70 S6k.Citation[30] As both studies applied sirolimus to ischemia-injured organs, these adverse effects are probably seen in conditions of IRI. In clinical trials, no serious nephrotoxic side effect was implicated. In this study, the increase in BUN and Cr levels associated with pretreatment could be due to the toxic effects of everolimus on tubular cells.
Myeloperoxidase, which shows inflammation, and MDA, the most important marker showing tissue injury in IRI, decreased in IR/eve group, indicating the protective effect of everolimus against inflammation and lipid peroxidation. In addition, the role of TNF-α has been clarified in renal IR, and everolimus decreases the TNF-α levels in IRI. Increase in IL-6 levels should be accepted as a positive effect of everolimus regarding the decrease in MPO, MDA, and TNF-α levels. The toxic effects of everolimus to the renal tubule cells cause an increase in renal functions, resulting in an inconsistency with the other findings.
In conclusion, we have demonstrated that pretreatment with everolimus had beneficial effects on cytokines, oxidative stress, and inflammatory markers in renal IRI. However, everolimus was not capable of attenuating renal functions and histopathological changes during renal IRI due to its potentially toxic effects on tubular cells.
ACKNOWLEDGMENTS
None of the authors reports any conflicts of interest, nor has any funding for this article been disclosed.
REFERENCES
- Bonventre JV, Zuk A. Ischemic acute renal failure: An inflammatory disease?. Kidney Int. 2004;66:480–485.
- Lameire N, Biesen WV, Vanholder R. Acute renal failure. Lancet. 2005;365:417–430.
- Sheridan AM, Bonventre JV. Pathophysiology of ischemic acute renal failure. Contrib Nephrol. 2001;132:7–21.
- Molitoris BA, Sutton TA. Endothelial injury and dysfunction: Role in the extension phase of acute renal failure. Kidney Int. 2004;66:496–499.
- Augustine JJ, Hricik DE. Experience with everolimus. Transplant Proc. 2004;36(Suppl. 2):500–503.
- Tan HP, Basu A, Shapiro R. Everolimus: An update. Curr Opin Organ Transplant. 2003;8:323–326.
- Schuurman HJ, Pally G, Weckbecker G, SDZ RAD inhibits cold ischemia-induced vascular remodeling. Transplant Proc. 1999;31:1024–1025.
- Lehle K, Birnbaum DE, Preuner JG. Predominant inhibition of interleukin-6 synthesis in patient-specific endothelial cells by mTOR inhibitors below a concentration range where cell proliferation is affected and mitotic arrest takes place. Transplant Proc. 2005;37:159–161.
- Puglisi RN, Strande L, Santos M, Beneficial effects of cyclosporine and rapamycin in small bowel ischemic injury. J Surg Res. 1996;65:115–118.
- Reis F, Parada B, Teixeira de Lemos E, Hypertension induced by immunosuppressive drugs: a comparative analysis between sirolimus and cyclosporine. Transplant Proc. 2009;41:868–873.
- Crowe A, Bruelisauer A, Duerr L, Absorption and intestinal metabolism of SDZ-RAD and rapamycin in rats. Drug Metab Dispos. 1999;27:627–632.
- Kirchner GI, Meier-Wiedenbach I, Manns MP. Clinical pharmacokinetics of everolimus. Clin Paharmacokinet. 2004;43:83–95.
- Okhawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358.
- Sun Y, Oberley LW, Li Y. A simple method for clinical assay of superoxide dismutase. Clin Chem. 1988;34:497–500.
- Lopez-Neblina F, Toledo-Pereyra LH, Mirmiran R, Time dependence of Na-nitroprusside administration in the prevention of neutrophil infiltration in the rat ischemic kidney. Transplantation. 1996;61:179–183.
- Sahna E, Parlakpinar H, Ozturk F, The protective effects of physiological and pharmacological concentrations of melatonin on renal ischemia-reperfusion injury in rats. Urol Res. 2003;31:188–193.
- Adkins JR, Silendirresena MR, Wang Z, Rapamycin inhibits release of tumor necrosis factor-α from human vascular smooth muscle cells. Am Surg. 2004;70:384–387.
- Donahoo KK, Shames BD, Harken AH, The role of tumor necrosis factor in renal ischemia-reperfusion injury. J Urol. 1999;162:196–203.
- Meldrum KK, Meldrum DR, Hile KL. p38MAPK mediates renal tubular cell TNF-α production and TNF-α dependent apoptosis during simulated ischemia. Am J Physiol Cell Physiol. 2001;281:563–570.
- Donnahoo KK, Meldrum DR, Shenkar R, Early renal ischemia, with or without reperfusion, activates NFkB and increases TNF-α bioactivity in the kidney. J Urol. 2000;163:1328–1332.
- Donnahoo KK, Meng X, Ao L, Differential cellular immunolocalization of renal tumor necrosis factor-α production during ischemia versus endotxaemia. Immunology. 2001;102:53–58.
- Patel NSA, Chatterjee PK, Di Paola R. Endogenous interleukin-6 enhances the renal injury, dysfunction, and inflammation caused by ischemia/reperfusion. J Pharmacol Exper Ther. 2005;312:1170–1178.
- Yokota N, O'Donnell M, Daniels F. Protective effect of HMG-CoA reductase inhibitor on experimental renal ischemia-reperfusion injury. Am J Nephrol. 2003;23:13–17.
- Baud L, Ardaillou R. Involvement of reactive oxygen species in kidney damage. Br Med Bull. 1993;49:621–629.
- Greene EL, Paller MS. Oxygen free radicals in acute renal failure. Miner Electrolyte Metab. 1991;17:124–132.
- Dobashi K, Ghosh B, Orak JK, Kidney ischemia-reperfusion: Modulation of antioxidant defenses. Mol Cell Biochem. 2000;205:1–11.
- Ysebaert DK, De Greef KE, Vercauteren SR. Identification and kinetics of leukocytes after severe ischaemia/reperfusion renal injury. Nephrol Dial Transplant. 2000;15:1562–1574.
- Daniel C, Renders L, Amann K, Mechanisms of everolimus-induced glomerulosclerosis after glomerular injury in the rat. Am J Transplant. 2005;5:2849–2861.
- Goncalves GM, Cenedeze MA, Feitoza CQ. The role of heme oxygenase 1 in rapamycin-induced renal dysfunction after ischemia and reperfusion injury. Kidney Int. 2006;70:1742–1749.
- Lieberthal W, Fuhro R, Andry CC. Rapamycin impairs recovery from acute renal failure: Role of cell-cycle arrest and apoptosis of tubular cells. Am J Physiol Renal Physiol. 2001;281:693–706.