Abstract
Aldosterone is reported to promote fibrosis of multiple organs. Recent studies showed that Na+-H+ exchanger isoform 1 (NHE1) was involved in mineralocorticoid-induced tissue fibrosis. The present study examined the role of NHE1 in aldosterone-induced glomerulosclerosis in rats. SD male rats were subjected to 5/6 nephrectomy and divided into four groups: rats subjected to sham operation were used as control (SHAM group), 5/6 nephrectomy (SNX group), SNX treated with aldosterone via osmotic mini-pump (ALDO group), and SNX treated with aldosterone plus NHE1 inhibitor 5-(N, N-Dimethyl) amiloride hydrochloride (DMA) (ALDO+DMA group). The rats were sacrificed at the 12th week. We found that aldosterone treatment significantly increased kidney weight/body weight ratio and systolic blood pressure compared with SNX rats. Aldosterone also increased proteinuria and serum creatinine level. The NHE1 antagonist DMA significantly reversed the effect of aldosterone on proteinuria, but had no effect on the aldosterone associated hypertension and the elevation of serum creatinine. The remnant kidney of 5/6 nephrectomized rats exhibited increased glomerulosclerosis score, tubulointerstitial fibrosis, and tubular proteinaceous cast, which were significantly enhanced by aldosterone treatment. DMA treatment significantly reduced aldosterone-associated glomerulosclerosis, but failed to improve aldosterone-induced tubulointerstitial fibrosis and tubular proteinaceous cast. The aldosterone-induced increase in renal TGFβ1 and PCNA was significantly prevented by treatment with DMA. Our data showed that NHE1 inhibitor reduced aldosterone-induced glomerulosclerosis but not hypertension in 5/6 nephrectomized rats. The present study suggested that NHE1 contributed to aldosterone-induced-glomerulosclerosis and could be a potential therapeutic target for chronic kidney disease.
INTRODUCTION
Accumulating clinical and experimental studies have demonstrated that the locally produced aldosterone played an important role in the pathogenesis of multiple tissue fibrosis and remodeling, independent of its effects on hemodynamic regulation and fluid homeostasis.Citation[1–3] Our previous studies have demonstrated that renal mesangial cell was a target of local aldosterone action, and aldosterone increased extra-cellular matrix (ECM) protein fibronectin expression, which was blocked by mineralocorticoid receptor antagonist spironolactone.Citation[4] Collectively, these data suggested that aldosterone has deleterious effects on the kidney that cannot be explained simply by systolic blood pressure (SBP) changes. The mechanisms underlying the pathophysiological actions of aldosterone in the kidney have not been completely defined.
Na+-H+ exchanger isoform 1 (NHE1) is a plasma membrane transport protein. In addition to the basic role of the pHi and volume regulation of the cell, recent studies suggested that NHE1 also had an important role in cell proliferation, division, or migration.Citation[5] Recent studies also showed that NHE1 can be activated by aldosterone and involved in aldosterone induced cardiac hypertrophy.Citation[6] These findings suggested that the aldosterone/NHE1 pathway plays a role in mediating renal injury. However, there is no convincing evidence to indicate the potential contribution of NHE1 to aldosterone-induced renal injury.
The purpose of this study, therefore, is to do the research on the NHE1-dependent glomerulosclerosis induced by aldosterone in vivo. To test our hypothesis, we investigated whether renal injury that was induced by chronic treatment with aldosterone was associated with activation of NHE1. Further studies were performed to test the effects of a specific NHE1 inhibitor 5-(N, N-Dimethyl) amiloride hydrochloride (DMA) on renal injury that is induced by long-term treatment with aldosterone. Because aldosterone has been suggested to induce tissue fibrosis through the activation of transforming growth factor beta-1 (TGFβ1)-dependent mechanism,Citation[7–10] we also studied the effects of DMA on the expression of renal TGFβ1 and fibronectin in aldosterone-treated rats. Proliferating cell nuclear antigen (PCNA) was also examined in aldosterone-treated rats to test the mechanism of cell proliferation in aldosterone-induced glomerulosclerosis and the effect of DMA on it.
MATERIALS AND METHODS
Animal Preparation
Five-week-old male Sprague-Dawley rats that weighed 180–210 grams at the beginning of the experiments underwent 5/6 renal ablation under anesthesia with sodium pentobarbital (50 mg/kg, intraperitoneally). Rats were treated for 12 weeks with one of the following:
vehicle: free access to saline, n = 8 (SNX group);
aldosterone (40 μg.kg–1 d): subcutaneously; n = 8; Sigma, USA (ALDO group); or
aldosterone (40 μg.kg–1 d) + DMA (100 μg.kg–1 d): subcutaneously; n = 7; Sigma, USA (ALDO+DMA group).
The rats of a sham group were also operated under the same condition as control (n = 8; SHAM group). An osmotic minipump (model 2004; Alza Co, Palo Alto, California, USA) was implanted subcutaneously to infuse the aldosterone and replaced every four weeks under sodium pentobarbital anesthesia. SBP was measured in conscious rats by tail-cuff plethysmography (BP-98A; Softron Co., Tokyo, Japan), and 24 h urine samples were collected at the 12th week. Blood and kidney samples were collected at the end of week 12 under sodium pentobarbital anesthesia. Kidneys were perfused with chilled saline solution, and then were fixed in 4% paraformaldehyde and embedded in paraffin for histologic examination. Remaining renal tissues were stored at −70°C until processing for protein extractions.
Histologic Examination
Kidneys were sectioned into 3 μm slices, and then stained with periodic acid-Schiff (PAS) and Masson's trichrome reagents. The severity of glomerular proliferation, interstitial fibrosis, and tubular proteinaceous cast scores were evaluated using light microscopy according to previously described methods.Citation[11–15] Briefly, glomerular proliferative lesions were scored into five grades as follows: 0, no proliferation; 1, minor (segmental lesion < 25%); 2, mild (segmental lesion 25–50%); 3, moderate (diffuse proliferation without severe sclerotic change); and 4, severe (diffuse proliferation with nearly complete sclerosis).Citation[11–13] A minimum of 40 glomeruli were examined in each PAS-stained specimen. The severity of tubulointerstitial fibrosis was assessed by counting the percentage of injured areas per field and then scored as follows: 0, normal interstitium; 0.5, < 5% of areas injured; 1, 5–15%; 1.5, 16–25%; 2, 26–35%; 2.5, 36–45%; and 3, > 45%.Citation[13,14] A minimum of 20 fields were evaluated for each Masson's trichrome-stained specimen. Tubular proteinaceous cast scores were scored into five grades as follows: 0, no damage; 1, mild (patchy isolated damage); 2, moderate (damage < 25%); 3, severe (damage 25–50%); and 4, very severe (damage > 50%). A minimum of 10 fields was evaluated for each Masson's trichrome-stained specimen. Proteinaceous cast-positive areas in tubuli (strong red) were also calculated, and these affected areas in turn were divided by the total tubular area of the microscopic field.Citation[12,15] We did this experiment with three sections of each kidney issue for the semiquantitative analysis. The above histologic analysis was performed in a blind manner to avoid bias.
Immunohistochemical Staining
The kidney cortex samples of male Sprague-Dawley rats were fixed with 4% paraformaldehyde for 6 h. Then the paraffin embed tissues were sliced into 3 μm-thick sections. The sections were deparaffinized with xylene and rehydrated with 100% and then 70% alcohol. The sections were then treated with 3% hydrogen peroxide/methanol to block endogenous peroxidase. Then the sections were incubated with monoclonal anti-rat TGFβ1 antibody, anti-rat fibronectin antibody (1:200; Santa Cruz Biotechnology, Inc., USA), and anti-rat PCNA antibody (1:200; Santa Cruz Biotechnology, Inc. USA), respectively, overnight at 4°C in a humidified chamber. The avidin-biotin-peroxidase complex (ABC-Elite, Vector Laboratories) was used as a detection system. Peroxidase activity was revealed by diaminobenzidine tetrahydrochloride. We did this experiment with three sections of each kidney issue for the semiquantitative analysis.
Western Blot
Total protein was extracted from the kidney cortex samples. Then the protein was electrophoresed on a 10% polyacrylamide gel and transferred to a Hybond-C nitrocellulose membrane. The expression of TGFβ1 was detected by Western blot using anti-rat TGFβ1 antibody (1:500, Santa Cruz Biotechnology, Inc. USA) at 4°C overnight. After washing, the membrane was incubated with peroxidase-conjugated goat anti-rabbit IgG (1:2000, Vector, USA) at room temperature for 1h. The expression of PCNA was detected by Western blot using rabbit anti-PCNA antibody raised against rat (1:500, Santa Cruz Biotechnology, Inc., USA) at 4°C overnight. The membrane was then incubated in peroxidase-conjugated goat anti-rabbit IgG (1:2000, Vector, USA) at room temperature for 1h. Immunoreactive proteins were visualized using enhanced chemiluminescence (ECL) (KPL, USA).
Statistical Analysis
The processing of statistical data was conducted using SPSS 15.0 software. The results were presented with mean ± SE. ANOVA was used to compare mean values. Significance was accepted when p < 0.05.
RESULTS
Body and Kidney Weight and SBP
The results of kidney weight/body weight ratio (KW/BW), BP, urinary excretion rate of protein, and plasma creatinine are depicted in . The KW/BW was much higher in rats that received aldosterone infusion than in rats that received vehicle infusion. Treatment with DMA didn't obviously decrease KW/BW in the rats that received aldosterone infusion (see ). The data of SBP was also shown in . Rats that underwent 5/6 renal ablation showed a higher SBP compared with animals of the SHAM group. Rats that received aldosterone infusion showed the highest SBP among all the groups, but treatment with DMA did not obviously alter the SBP of rats that received aldosterone infusion.
Table 1 Effects of aldosterone and DMA on KW/BW, SBP, urinary excretion rate of protein, and serum creatinine in 5/6 nephrectomy rats at the 12th week
Urinary Excretion Rate of Protein and Plasma Creatinine
As described in , aldosterone infusion resulted in severe proteinuria, and treatment with DMA markedly reduced urinary excretion rate of protein in rats that received aldosterone infusion. Rats that received aldosterone infusion showed increased plasma creatinine levels compared with rats of the SNX group. Treatment with DMA did not prevent aldosterone-induced changes in plasma creatinine levels (see ).
Histologic Findings
The glomerular histologic findings with PAS staining at week 12 are shown in through , and renal cortical and medullary histologic findings with Masson's trichrome staining are illustrated in through . respectively. Rats of the SHAM group showed the normal glomeruli (see ) and tubulointerstitium (see ); however, rats that received 5/6 renal ablation showed severe glomerular sclerosis (see ), and severe tubulointerstitial fibrosis and proteinaceous casts also were observed in the SNX group. Furthermore, rats that received aldosterone infusion exhibited the most injured glomeruli characterized by cell proliferation and sclerosis than the SNX group (see ). The most severe tubulointerstitial fibrosis and proteinaceous casts were also observed in the ALDO group (see ). Treatment with DMA significantly attenuated aldosterone-induced glomerular sclerosis (see ), but did not obviously attenuate aldosterone-induced tubulointerstitial fibrosis and proteinaceous casts (see ).
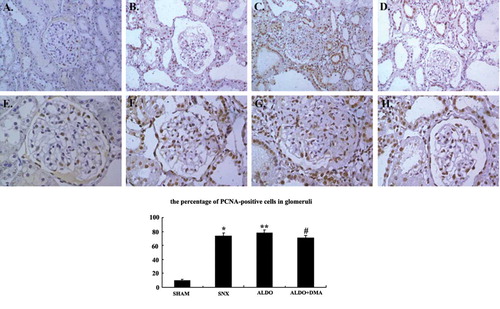
Figure 1. Photomicrographs of glomeruli (A–D). Glomerular proliferation was evaluated as described as E. In renal ablation rats, glomerular sclerosis was dramatically more serious than that of the SHAM group (B vs. A). Rats that received aldosterone infusion exhibited more serious glomerular sclerosis than the 5/6 nephrectomy rats (C vs. B). Treatment with DMA significantly attenuated aldosterone-induced glomerular injury (D). A: SHAM group; B: SNX group; C: ALDO group; D: ALDO+DMA group. *p < 0.01 versus SHAM group; **p < 0.01 versus SNX group; #p < 0.01 versus ALDO group. Magnification: ×400, PAS staining.
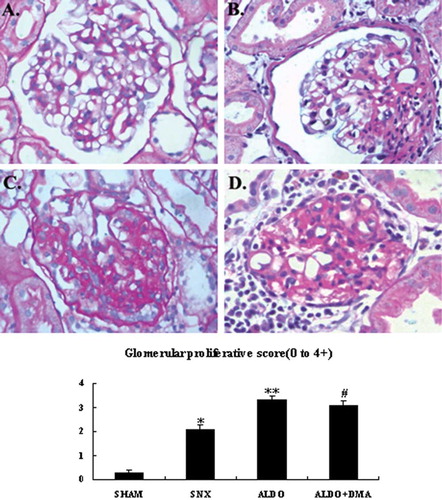
Figure 2. Photomicrographs of tubulointerstitium. Tubulointerstitial fibrosis and proteinaceous casts in tubuli were evaluated as described as E. In renal ablation rats, interstitial fibrosis and proteinaceous casts in tubuli was dramatically more serious than that of the SHAM group (B vs. A). Rats that received aldosterone infusion exhibited more serious interstitial fibrosis and proteinaceous casts in tubuli than the 5/6 nephrectomy rats (C vs. B). Treatment with DMA had no significant effect on the aldosterone-induced interstitial fibrosis and proteinaceous casts in tubuli (D). A: SHAM group; B: SNX group; C: ALDO group; D: ALDO+DMA group. *p < 0.01 versus SHAM group; **p < 0.05 versus SNX group. Magnification: ×400, Masson's trichrome.
Effect of DMA on Aldosterone-Induced Fibronectin, TGFβ1, and PCNA Expression by Immunohistochemistry
Renal cortical immunohistochemistry with fibronectin antibody at week 12 was illustrated in through , respectively. Renal cortical immunohistochemistry with TGFβ1 antibody was illustrated in through . And we also demonstrated the expression of PCNA in renal cortex in through . In rats that received 5/6 renal ablation, fibronectin- and TGFβ1-positive cells were observed in glomerular mesangial spaces and renal interstitial spaces (see and ). PCNA-positive cells were observed in the cell nuclei of both glomeruli and interstitium (see ). However, in rats that received aldosterone infusion, renal injury was associated with significant increases in the numbers of fibronectin-, TGFβ1-, and PCNA-positive cells (see , , and ) in the renal cortex. The number of TGFβ1- and PCNA-positive cells in the renal cortex was much less in aldosterone- and DMA-treated rats compared with rats that were treated with aldosterone alone (see and ).
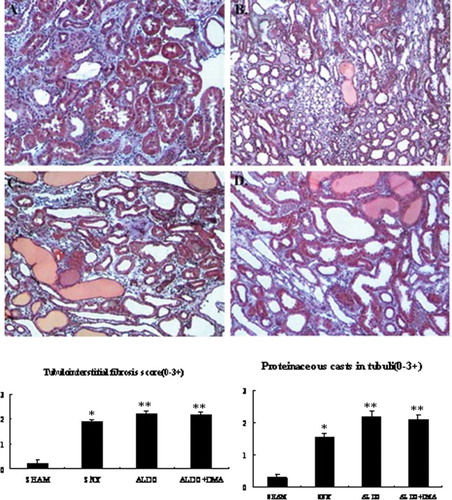
Figure 3. Representative photographs of immunohistochemistry for fibronectin. In renal ablation rats, fibronectin was observed slightly in the glomerular mesangium and the renal interstitium (B). However, in rats that received aldosterone infusion, renal injury was associated with increases in the numbers of fibronectin-positive (C and D) cells in the glomerular mesangium and the renal interstitium. There was no significant difference between aldosterone DMA-treated rats and the rats that were treated with aldosterone alone (C and D). A: SHAM group; B: SNX group; C: ALDO group; D: ALDO+DMA group. *p < 0.01 versus SHAM group; **p < 0.05 versus SNX group. Magnification: ×400.
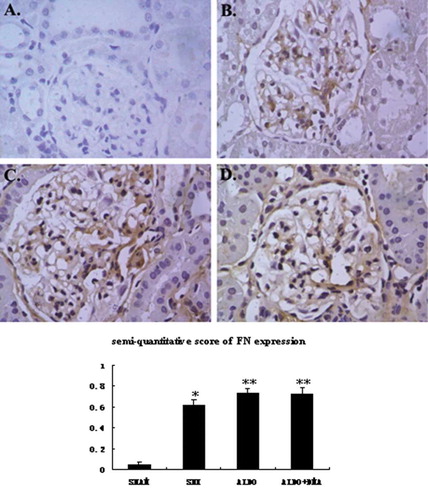
Figure 4. Representative photographs of immunohistochemistry for TGFβ1. In rats that received 5/6 nephrectomy, TGFβ1 was observed slightly in the glomerular mesangium and the renal interstitium (B). However, in rats that received aldosterone infusion, renal injury was associated with increases in the numbers of TGFβ1-positive (C and D) cells. The numbers of TGFβ1-positive cells seemed to be much less in aldosterone DMA-treated rats compared with rats that were treated with aldosterone alone (C and D). A: SHAM group; B: SNX group; C: ALDO group; D: ALDO+DMA group. *p < 0.01 versus SHAM group; **p < 0.05 versus SNX group; #p < 0.05 versus ALDO group. Magnification: ×400.
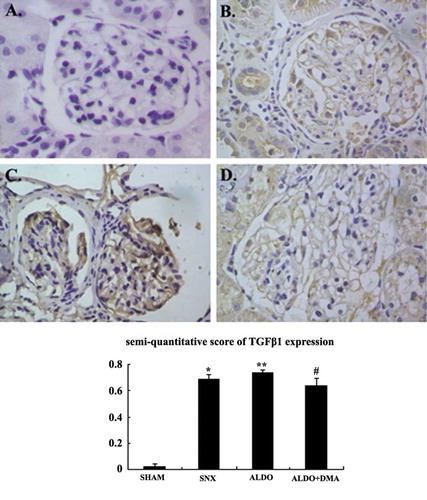
Figure 5. Representative photographs of immunohistochemistry for PCNA. In rats that received 5/6 nephrectomy, PCNA was expressed slightly higher in nuclei of the glomeruli and renal tubuli than that of the SHAM group rats (B and F). But in rats that received aldosterone infusion, cell proliferation was associated with dramatic increases in the numbers of PCNA-positive (C and G) cells. The numbers of PCNA-positive cells seemed to be much less in aldosterone and DMA–treated rats compared with rats that were treated with aldosterone alone (D and H). A and E: SHAM group; B and F: SNX group; C and G: ALDO group; D and H: ALDO+DMA group. *p < 0.01 versus SHAM group; **p < 0.05 versus SNX group; #p < 0.05 versus ALDO group. Magnification: A–D, ×200; E–H, ×400.
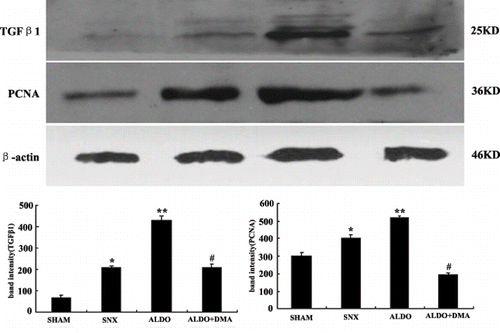
Effect of DMA on Aldosterone-Induced TGFβ1 and PCNA Expression by Western Blot
As shown in , the levels of TGFβ1 and PCNA in renal cortical tissues were markedly increased in rats that received 5/6 renal ablation than the SHAM group (see ). Furthermore, the aldosterone infusion revealed even higher levels of TGFβ1 and PCNA (see ). The aldosterone-induced increase in renal TGFβ1 and PCNA was prevented by treatment with DMA (see ), which was consistent with the results of immunohistochemistry.
Figure 6. The expression of TGFβ1 and PCNA in renal cortical tissues by Western blot. TGFβ1 and PCNA in renal cortical tissues were markedly increased in rats that received aldosterone infusion, indicating ECM accumulation and cell proliferation in the kidney. The aldosterone-induced increase in renal cortical TGFβ1 and PCNA was prevented by treatment with DMA. Data are expressed as the relative differences between SNX rats and ALDO or ALDO+DMA treated rats after normalization to β-actin expression. *p < 0.01 versus SHAM group; **p < 0.01 versus SNX group; #p < 0.05 versus ALDO group.
DISCUSSION
Reversing or slowing down the process of glomerulosclerosis has always been one of the most important issues among patients with chronic kidney diseases. Removal of 5/6 of the renal mass resulted in the progression of renal fibrosis, which is a classical model of glomerulosclerosis.Citation[16] The results of our study demonstrated that the remnant kidney weight and the kidney weight/body weight ratio of the rats that received 5/6 renal ablation were much higher compared with animals of the SHAM group. Rats that underwent 5/6 renal ablation showed a higher SBP and more severe proteinuria and plasma creatinine compared with animals of the SHAM group. Furthermore, severe glomerulosclerosis, tubulointerstitial fibrosis, and proteinaceous casts were also observed in the rats of the SNX group. Consistent with previous studies of the remnant nephropathy in the rats,Citation[17] our data indicated that we successfully constructed the 5/6 renal ablation and progressive glomerulosclerosis animal model.
In most patients with chronic kidney disease, plasma aldosterone concentration is increased and correlated with renal dysfunction.Citation[18] Fredersdorf et al. showed that in a model of type 2 diabetes mellitus with known diabetic nephropathy and cardiac remodeling, urinary and plasma aldosterone levels were elevated.Citation[19] Renin-angiotensin-aldosterone system (RAS) inhibition and aldosterone blockade has been associated with decreased proteinuria and glomerulosclerosis in patients with chronic kidney diseases and reduced renal damage.Citation[20,21] Terada et al. showed their results that the SGK1–NF-κB pathway played a key role in glomerular fibrosis and inflammation in vivo and in vitro.Citation[22] However, the mechanisms underlying the pathophysiological actions of aldosterone in the kidney have not been completely defined.
NHE is a ubiquitous protein present in mammalian cells. NHE is composed of a membrane bound domain of approximately 500 amino acids plus a hydrophilic regulatory cytoplasmic domain of approximately 315 amino acids. NHE1 is the first discovered and the best characterized isoform of the NHE family,Citation[23] which consists of ten known isoforms. NHE1 is participated in certain physiological and pathological processes, such as cell proliferation, growth, migration and apoptosis in normal condition, as well as cancer cell invasion and heart failure.Citation[5] Recently, NHE1 inhibition has been shown to be remarkably effective in preventing hypertrophy and fibrosis in some animal models.Citation[6,24] Whether this proves to be a practical treatment for hypertrophy or other target organ injury has yet to be determined.
In addition, the enhanced erythrocyte NHE1 activity was indicated to be associated with left ventricular hypertrophy and elevated plasma aldosterone levels in hypertensive patients.Citation[25] Studies have also showed that NHE1 was involved in aldosterone induced cardiac hypertrophy in neonatal rat ventricular myocytes.Citation[26] NHE1 was recently found to be activated by aldosterone in vascular smooth muscle cells and proximal tubular cells.Citation[27,28] However, most of these researches were in vitro and the mechanism by which aldosterone activated NHE1 has not been elucidated till now. Based on the possible link between aldosterone and NHE1 in renal damage, we performed our research on the NHE1-dependent glomerulosclerosis induced by aldosterone in vivo.
Our study provided evidence that long-term administration of aldosterone to rats that underwent 5/6 renal ablation induced higher hypertension and more severe renal injury compared with the rats of the SNX group. Renal fibrosis is characterized mainly by the ECM accumulation, cell proliferation and the hypertrophy of the kidney.Citation[17] We have found that aldosterone-induced renal injury is associated with increases in the expression of fibronectin, one of the ECM components and TGFβ1, the important cytokine involved in the fibrogenesis. In addition, the higher levels of PCNA were also observed in the aldosterone treated rats. Infusion of DMA, the antagonist of NHE1, did not alter BP but markedly attenuated the progression of glomerulosclerosis in these rats. Our results demonstrated that NHE1 contributed to the progression of aldosterone-induced glomerulosclerosis in the rat through the mechanisms of more than SBP. DMA ameliorated remnant nephropathy in the rats via inhibiting the ECM accumulation and the cell proliferation.
Excessive synthesis and accumulation of ECM components is one of the key mechanisms of the progression of renal fibrosis.Citation[29] Consistent with the results of previous studies in vivo and in vitro, chronic treatment with aldosterone resulted in the ECM synthesis of different target organs, including heart, vascular, and kidney [Citation[4,7,30,31] It is well documented that TGFβ1 is a signaling pathway mediating ECM protein, including fibronectin expression.Citation[32–35] Studies showed that activation of MR by aldosterone is associated with activation of TGFβ1 signaling, Citation[7–10] which can be inhibited by the MR antagonist eplerenone. We also revealed that the inhibition of NHE1 with DMA strongly suppressed the high expression of TGFβ1 induced by aldosterone. At the same time, proteinuria and glomerulosclerosis were also improved by the inhibition of NHE1. In summary, these data suggested that at least some of the renoprotective effects of NHE1 inhibitor on aldosterone-induced renal injury were mediated by inhibiting the ECM accumulation.
PCNA is a nuclear protein that is expressed from late G1 through the M phase of the cell cycle.Citation[36] PCNA expression has been shown by immunostaining to be elevated in ANG II-infused Sprague-Dawley rats.Citation[37] Peng et al. showed that six weeks of treatment with aldosterone significantly increased interstitial and perivascular PCNA-positive cells in the left ventricle and kidney.Citation[38] In our study, aldosterone-induced renal fibrosis was associated with increases in PCNA expression by immunochemistry and Western blot. Moreover, NHE1 inhibition with DMA did not alter SBP but markedly attenuated aldosterone-induced augmentation of PCNA expression and renal fibrosis, suggesting that NHE1 was involved in aldosterone-induced proliferation of renal cells, possibly contributing to the progression of renal fibrosis.
The present study suggested that NHE1 played an important role in aldosterone-mediated renal fibrosis via inhibiting ECM accumulation and cell proliferation in 5/6 nephrectomized rats. Our results also showed that NHE1 antagonist reduced aldosterone induced glomerulosclerosis but not hypertension in 5/6 nephrectomized rats. In conclusion, the data of our research demonstrated that NHE1 contributed to aldosterone-induced glomerulosclerosis, which could be a potential therapeutic target for chronic kidney disease.
ACKNOWLEDGMENTS
This work was supported by National Nature Science Foundation of China 30670977 (to Yong Gu) and 30900685 (to Minmin Zhang), Key Project of Basic Research by Science and Technology Commission of Shanghai Municipality and 07JC14007 (to Yong Gu).
REFERENCES
- Stier CTJr, Rocha R, Chander PN. Effect of aldosterone and MR blockade on the brain and the kidney. Heart Fail Rev. 2005;10:53–62.
- Marney AM, Brown NJ. Aldosterone and end-organ damage. Clin Sci. 2007;113(6):267–278.
- Young MJ. Mechanisms of mineralocorticoid receptor-mediated cardiac fibrosis and vascular inflammation. Curr Opin Nephrol Hypertens. 2008;17:174–180.
- Lai L, Chen J, Hao CM, Lin S, Gu Y. Aldosterone promotes fibronectin production through a Smad2-dependent TGF-β1 pathway in mesangial cells. Biochem Biophys Res Comm. 2006;348:70–75.
- Karmazyn M, Sawyer M, Fliegel L. The Na+/H+ exchanger: a target for cardiac thearapeutic intervention. Current Drug Targets—Cardiovascular and Haematological Disorders. 2005;5:323–335.
- Baartscheer A, Schumacher CA, van Borren MM, Belterman CN, Chronic inhibition of Na+/H+-exchanger attenuates cardiac hypertrophy and prevents cellular remodeling in heart failure. Cardiovasc Res. 2005;65:83–92.
- Huang W, Xu C, Kahng KW, Noble NA, Border WA, Huang Y. Aldosterone and TGF-β1 synergistically increase PAI-1 and decrease matrix degradation in rat renal mesangial and fibroblast cells. Am J Physiol Renal Physiol. 2008;294:F1287–F1295.
- Yuan J, Jia R, Bao Y. Beneficial effects of spironolactone on glomerular injury in streptozotocin-induced diabetic rats. J Renin Angiotensin Aldosterone Syst. 2007;8:118–126.
- Nishioka T, Suzuki M, Onishi K, Takakura N, Eplerenone attenuates myocardial fibrosis in the angiotensin II-induced hypertensive mouse: Involvement of tenascin-C induced by aldosterone-mediated inflammation. J Cardiovasc Pharmacol. 2007;49:261–268.
- Chun TY, Chander PN, Kim JW, Pratt JH, Stier CTJr.. Aldosterone, but not angiotensin II, increases profibrotic factors in kidney of adrenalectomized stroke-prone spontaneously hypertensive rats. Am J Physiol Endocrinol Metab. 2008;295:E305–E312.
- Nagai Y, Yao L, Kobori H, Temporary angiotensin II blockade at the prediabetic stage attenuates the development of renal injury in type 2 diabetic rats. J Am Soc Nephrol. 2005;16:703–711.
- Fan Y-Y, Baba R, Nagai Y, Augmentation of intrarenal angiotensin II levels in uninephrectomized aldosterone/salt-treated hypertensive rats: Reno-protective effects of the ultrahigh dose of olmesartan. Hypertens Res. 2006;29:169–178.
- Kobori H, Ozawa Y, Suzaki Y, Nishiyama A. Enhanced intrarenal angiotensinogen contributes to early renal injury in spontaneously hypertensive rats. J Am Soc Nephrol. 2005;16:2073–2080.
- Li C, Yang CW, Park CW, Long-term treatment with ramipril attenuates renal osteopontin expression in diabetic rats. Kidney Int. 2003;63:454–463.
- Teraishi K, Kurata H, Nakajima A, Takaoka M, Matsumura Y. Preventive effect of Y-27632, a selective Rho-kinase inhibitor, on ischemia/reperfusion-induced acute renal failure in rats. Eur J Pharmacol. 2004;505:205–211.
- Anderson S, Meyer TW, Rennke HG, Brenner BM. Control of glomerular hypertension limits glomerular injury in rats with reduced renal mass. J Clin Invest. 1985;76:612–619.
- Lax DS, Benstein JA, Tolbert E, Effects of salt restriction on renal growth and glumerular injury in rats with remnant kidneys. Kidney Int. 1992;41:1527–1534.
- Hené RJ, Boer P, Koomans HA, Mees EJ. Plasma aldosterone concentrations in chronic renal disease. Kidney Int. 1982;21:98–101.
- Fredersdorf S, Endemann DH, Luchner A, Increased aldosterone levels in a model of Type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes. 2009;117:15–20.
- Sato A, Hayashi K, Naruse M, Saruta T. Effectiveness of aldosterone blockade in patients with diabetic nephropathy. Hypertension. 2003;41:64–68.
- Hou FF, Zhang X, Zhang GH, Efficacy and safety of benazepril for advanced chronic renal insufficiency. N Engl J Med. 2006;354:131–140.
- Terada Y, Kuwana H, Kobayashi T, Aldosterone-stimulated SGK1 activity mediates profibrotic signaling in the mesangium. Journal of the American Society of Nephrology. 2008;19:298–309.
- Harguindey S, Orive G, Luis Pedraz J, Paradiso A, Reshkin SJ. The role of pH dynomics and the Na+/H+ antiporter in the etiopathogenesis and treatment of cancer. Two faces of the same coin—one single nature. Biochimica et Biophysica Acta. 2005;1756:1–24.
- Baartscheer A, Hardziyenka M, Schumacher CA, Chronic inhibition of the Na+/H+ - exchanger causes regression of hypertrophy, heart failure, and ionic and electrophysiological remodelling. Br J Pharmacol. 2008;154:1266–1275.
- Navarro-Lopez F, Coca A, Pare JC, Left ventricular hypertrophy in asymptomatic essential hypertension: Its relationship with aldosterone and the increase in sodium-proton exchanger activity. European Heart Journal. 1993;14:38–41.
- Karmazyn M, Liu Q, Gan XT, Brix BJ, Fliegel L. Aldosterone increases NHE-1 expression and induces NHE-1-dependent hypertrophy in neonatal rat ventricular myocytes. Hypertension. 2003;42:1171–1176.
- Ebata S, Muto S, Okada K, Aldosterone activates Na+/H+ exchange in vascular smooth muscle cells by nongenomic and genomic mechanisms. Kidney Int. 1999;56:1400–1412.
- Pinto V, Pinho MJ, Hopfer U, Jose PA, Soares-da-Silva P. Oxidative stress and the genomic regulation of aldosterone-stimulated NHE1 activity in SHR renal proximal tubular cells. Mol Cell Biochem. 2008;310:191–201.
- Nangaku M. Mechanisms of tubulointerstitial injury in the kidney: Final common pathways to end-stage renal failure. Intern Med. 2004;43:9–17.
- Takeda M, Tatsumi T, Matsunaga S, Spironolactone modulates expressions of cardiac mineralocorticoid receptor and 11β-hydroxysteroid dehydrogenase 2 and prevents ventricular remodeling in post-infarct rat hearts. Hypertens Res. 2007;30:427–437.
- Funder JW, Mihailaidou AS. Aldosterone and mineralocorticoid receptors: Clinical studies and basic biology. Mol Cell Endocrinol. 2009;301:2–6.
- Kagami S, Border WA, Miller DE, Noble NA. Angiotensin II stimulates extracellular matrix protein synthesis through induction of transforming growth factor-β expression in rat glomerular mesangial cells. J Clin Invest. 1994;93:2431–2437.
- Anderson PW, Zhang XY, Tian J, Insulin and angiotensin II are additive in stimulating TGF-β1 and matrix mRNAs in mesangial cells. Kidney Int. 1996;50:745–753.
- Uchiyama-Tanaka Y, Matsubara H, Mori Y, Involvement of HB-EGF and EGF receptor transactivation in TGF-β-mediated fibronectin expression in mesangial cells. Kidney Int. 2002;62:799–808.
- Guo B, Koya D, Isono M, Sugimoto T, Kashiwagi A, Haneda M. Peroxisome proliferator-activated receptor-c ligands inhibit TGF-β1-induced fibronectin expression in glomerular mesangial cells. Diabetes. 2004;53:200–208.
- Reiss K, Meggs LG, Li P, Olivetti G, Capasso J, Anversa P. Upregulation of IGF1, IGF1-receptor, and late growthrelated genes in ventricular myocytes acutely after infarction in rats. J Cell Physiol. 1994;158:160–168.
- Johnson RJ, Alpers CE, Yoshimura A, Renal injury from angiotensin II-mediated hypertension. Hypertension. 1992;19:464–474.
- Peng H, Carretero OA, Raij L, Yang F, Kapke A, Rhaleb NE. Antifibrotic effects of N-acetyl-seryl-aspartyl-Lysyl-proline on the heart and kidney in aldosterone-salt hypertensive rats. Hypertension. 2001;37:794–800.
