Abstract
Cytoresistance is the term used to describe the response of the proximal tubule cells to various stress inducers via cholesterol accumulation. However, the role of extensive exercise as a renal insult has not been examined. In this study, the effect of heavy muscle activity on proximal tubule cytoresistance was investigated. Results obtained from rats subjected to running a treadmill for five days were compared to those of controls. Extensive muscle activity-induced soleus citrate synthase and blood lactate elevation were associated with normal MAP, RBF, and GFR. Blood electrolytes and cholesterol levels remained unchanged, whereas the total and free cholesterol accumulations in the proximal tubule cells of the exercised group were higher than controls. Cholesterol-loaded tubules were more resistant (as proved by LDH release) to an ATP-depleted/calcium overloaded second stress. These data clearly demonstrate that heavy muscle activity induces cholesterol accumulation in the proximal tubules of kidney, without influencing ATP generation.
Keywords:
INTRODUCTION
It is well known that exercise results in a significant redistribution of tissue blood flow, such that it is increased in the working muscles but decreased in uninvolved organs. Among these distant uninvolved organs, kidneys have a crucial importance. An exercising body needs normal kidney functions for the elimination of exercise-induced elevated metabolic waste products. Despite conflicted reports,Citation[1,2] most of the research studying renal blood flow suggests that each period of exercise creates an ischemia/reperfusion condition for kidneys when reduced kidney blood flow due to exercise returns to normal following exercise.Citation[3–8] In addition to its ischemia/reperfusion-like effects, strenuous muscle activity induces oxidative stress as measured by oxidative damage of lipids, proteins, and even genetic materials responsible for altered enzyme activities.Citation[9] Exercise-induced elevated oxidative stress has been also shown to increase apoptosis in the renal tubule cells.Citation[3]
There is clinical evidence supporting the results obtained from these experimental studies.Citation[10–13] Since 1936, it has been known that violent exercise is associated with a modification of kidney functions characterized by diminution of excretory rate, increased urinary pH, and the appearance of abnormal constituents in the urine.Citation[14] It is generally accepted that exercise intensity, rather than duration, has a more extensive effect on protein excretion rates. According to Poortmans and Labilloy,Citation[15] moderate or submaximal exercise affects mainly the glomerular structures, while strenuous exercise has an impact at both glomerular and tubular levels. Glomerular membrane permeability and the S1- S2 segments of proximal tubule cells seem to be the nephron parts that are most sensitive to biochemical changes related to the intensity of exercise.Citation[15,16]
Today, irrespective of their age and kidney function, people are encouraged to take regular muscular activity as part of a healthier lifestyle.Citation[17–19] Thus, the results of the above-mentioned experimental and clinical studies, indicating renal dysfunctions during heavy exercise,Citation[10–13] seem to be disregarded. One reason for this is the presence of lower blood creatinine levels and normal glomerular filtration rates (GFR) in regular exercisers.Citation[20,21] Another reason is the acceptance of the elevated protein excretion, which is observed after vigorous exercise, as a non-pathological proteinuria.Citation[22] In addition, several authors have attributed the unaltered kidney functions to the unidentified adaptive metabolic and functional changes induced by endurance exercise training.Citation[3,23–25] Taken together, these training-induced adaptive changes and the presence of normal kidney functions in training athletesCitation[26] may be the reason for the encouragement of regular exercise, ignoring the potentially damaging effects of heavy muscle activity on the kidneys.
The characteristics of endurance exercise-induced adaptive metabolic and functional changes in kidneys are not clear, but may include a cytoresistance-like response. In their numerous studies, Zager et al. have shown that when proximal tubule cells are subjected to diverse forms of injury such as acute renal failure, endotoxemia, ischemia/reperfusion injury, LPS insult, or oxidative stress, they undergo adaptive changes that serve to protect them from subsequent attacks.Citation[27–33] They denote these adaptive changes as acquired cytoresistance, which is generally expressed by 18 to 24 hrs following the initial insult. In these studies, heavy muscle activity or exercise is not included among the stress factors that initiate cytoresistance in proximal tubule cells. However, because kidney blood flow is reduced during exercise and recovers subsequently, strenuous exercise can potentially mimic ischemia/reperfusion injury, which may cause cytoresistance in the proximal tubule cells. It is entirely possible that the adaptive changes seen in trained athletes occur through this cytoprotection mechanism.Citation[3,23–25]
However, there is no clear consensus on the benefits or risks of acute and chronic exercise on kidney functions. Therefore, assessment of the effects of heavy physical exercise on the physiology and structure of proximal tubule cells is studied. This is an important clinical question given the encouragement by the health professionals for regular exercise at least five days per week.Citation[26] Even elderly people who are taking medications are encouraged to take up regular exercise to prevent age-induced kidney damage.Citation[34]
Therefore, we conducted an experimental study in rats that were subjected to heavy muscle activity in order to assess whether exercise can induce stress in proximal tubule cells and initiate acquired renal cytoresistance.
MATERIALS AND METHODS
2.5–3-month-old male Wistar rats weighing 286.92 ± 7.04 g were randomly divided into two groups. Twenty animals were used as a sedentary control, while the other 20 animals were subjected to heavy muscle activity for five days. The rats were provided food and water ad libitum. Post-exercise weight of the exercising animals remained unchanged (272.31 ± 6.62 g). All procedures were approved by Akdeniz University Animal Care and Usage Committee (06-12/02).
Exercise Protocol
In order to mimic the effects of irregular heavy muscle activity on kidney functions of laborers, short-term exhausting muscle activity was chosen as an exercise model. Animals were exhausted on a motor-driven treadmill (MAY-TME 9805, Commat, Ankara, Turkey) once a day for five days. Before the exhaustive protocol, the rats were familiarized with a treadmill running for two days. The exhaustive protocol was started at 20 m/min and a 5% gradient for 5 min. The gradient and speed were gradually increased to 15% and 24 m/min, and running was continued until exhaustion. For the animals that failed to escape from electrical stimulation, the exhaustion was approved by the loss of the straightening reflex for ten seconds when animals were placed on their back.
All animals were placed in metabolic cages for 24 hours, and urine samples were collected. The exhausted rats were placed in metabolic cages at the fourth exercise day and sacrificed after the last treadmill running. The urine samples were used for protein, glucose, and creatinine measurements. Protein, creatinine, and glucose levels were determined using the Lowry method,Citation[35] Jaffe method,Citation[36] and a commercial kit (QuantiChrom™ Glucose Assay Kit, DIGL-200), respectively. Urine and serum electrolyte levels were measured by an autoanalyzer (Roche Hitachi F-800).
Blood Lactate Concentration
Ten minutes following the last exercise session, lactate concentration was measured in blood samples obtained from the tail vein using a lactate analyzer BM-Lactate test strips (Accusport, Mannheim Boehringer, Accusport Diagnostics & Biochemicals, U.K.).
Activity of Citrate Synthase (CS)
CS activity was determined for the soleus muscle of each rat according to the spectrophotometric method described by Srere.Citation[37] Data were expressed as micromoles per gram wet weight per minute.
Measurement of Main Arterial Pressure (MAP)
Under light ether anesthesia, MAP was determined by tail cuff method (Biopac, BP HR200 module plus MP100 system, Goleta, California, USA).
Measurement of Renal Blood Flow (RBF)
Renal plasma flow (RPF) was calculated as the clearance of para-aminohippurate (PAH), and RBF was calculated as the clearance of RPF/(1 - Hct). Under the urethane anesthesia (1 mg/kg bw, ip), the left jugular vein and urinary bladder were cannulated for PAH infusion (0.6%) and urine collection, respectively. Plasma and urine PAH concentrations were determined by spectrophotometry.Citation[38,39]
After the collection of urine and blood samples, kidneys were perfused with ice-cold krebs phosphate buffer (118 mM NaCl, 4.8 mM KCl, 2.5 mM CaCl2, 1.2 mM MgSO4, 1.2 mM KH2PO4, 24 mM NaHCO3, 11 mM glucose, pH:7.4) to wash off the blood. Then, both kidneys were excised, cleaned from adhesive fat tissues, and decapsulated in an ice-cold buffer. The cooled kidneys were longitudinally dissected, and the medullary part was discarded. The renal cortical tissue was used for proximal tubule or mitochondria isolation.
Proximal Tubule Isolation
As previously described,Citation[40] renal proximal tubules were isolated from rats based on the method of Vinay et al.Citation[41] Briefly, the digested tissue was separated by centrifugation into four distinct bands, F1–F4. The proximal tubule-rich F4 band, after washing three times, was resuspended in krebs buffer and used for cholesterol determination or cytoresistance experiments.
Cholesterol Determination
Following isolation, proximal tubules were subjected to lipid extraction as previously described.Citation[28] Then, total and free cholesterol (FC) levels were determined using a commercial kit (Cholesterol Fluorometric (Red) Assay Kit, Amplex® Red from Molecular Probes, Invitrogen, A12216). Cholesterol ester (CE) level in samples was calculated by subtracting the free fraction from the total cholesterol. Serum total cholesterol and HDL cholesterol levels (following phosphotungstic acid/magnesium chloride precipitation) were determined using the same cholesterol kit. LDL cholesterol levels were calculated by subtracting the HDL cholesterol from total cholesterol.
Cytoresistance
As outlined by Zager et al.,Citation[28–30] isolated proximal tubules were incubated either in basal conditions or ATP-depleted/Ca+2 overloaded conditions, containing mitochondrial respiration and glycolysis inhibitors and Ca+2 ionophore (7.5 μM antimycin A, 20 mM 2-deoxyglucose and 10 μM A23187, respectively) for four hours. Medium LDH (lactate dehydrogenase) levels were determined using a commercial kit (QuantiChrom™ Lactate Dehydrogenase Kit, DLDH-100), and its release was expressed as a percentage of total LDH level.
Mitochondrial Isolation
Mitochondria were isolated from renal cortical tissue using a method defined by Weinberg et al.Citation[42] Isolated mitochondria were used for inorganic phosphate determination.
Inorganic Phosphate Determination
As an indicator of F0/F1-ATPase activity, inorganic phosphate levels in the mitochondria and PTSs were measured. This spectrophotometric method was previously described by Katewa and Katyare.Citation[43] Data were expressed as μg.mg prot–1.min–1.
Statistical Analysis
All values are presented as means ± SEM. Statistical comparisons were performed by unpaired Student's t-test. p < 0.05 was considered statistically significant.
RESULTS
In order to attain heavy muscle activity, the rats were exercised to exhaustion for five days, and the signs of cytoresistance were evaluated by measuring the tubular cholesterol and LDH release from the isolated proximal tubule segments. As shown in , five days of exhaustive muscle activity had no significant effect on MAP, GFR, or RBF. The blood total cholesterol, LDL-HDL cholesterol, and electrolytes were within normal limits. Fractionated sodium (FENa+) and potassium (FEK+) excretion were also unchanged.
Table 1 Studied parameters in rats from sedentary control and five-day exhausted groups
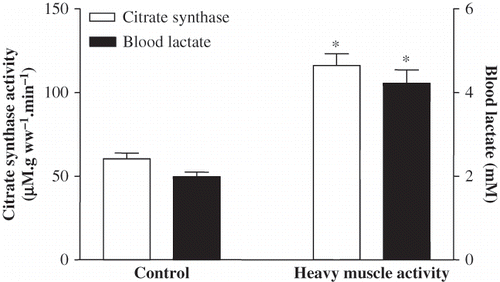
Despite normal GFR, the heavily exercised animals exhibited a significant proteinuria and glucosuria (see ). In the exhausted group, the mean blood lactate level was increased two-fold (from 1.99 ± 0.11 to 4.22 ± 0.32 mM, p < 0.001), and CS activity in soleus muscle was elevated significantly from 60.38 ± 3.45 μM. g ww–1. min–1 to 116.17 ± 6.98 μM. g ww–1. min–1 (p < 0.001; see ).
Figure 1. CS activity for soleus muscle and blood lactate levels in the sedentary control and in five-day exhausted animals (difference from sedentary control; *p < 0.001).
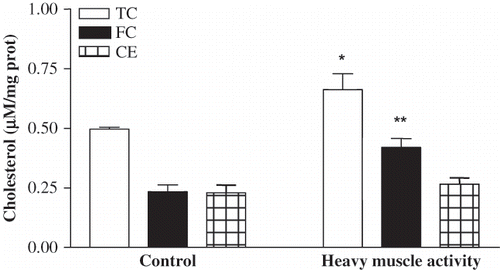
The isolated proximal tubule segments exhibited a different picture when cholesterol levels were considered (see ). Despite the lack of alteration in plasma cholesterol levels, the isolated proximal tubule segments from the exhausted rats revealed a significant increase in cholesterol levels with respect to the controls (0.662 ± 0.067 μM/mg prot vs. 0.499 ± 0.008 μM/mg prot; p < 0.05). The elevation was prominent in the free cholesterol fraction, increasing from 0.234 ± 0.029 μM/mg prot to 0.420 ± 0.037 μM/mg prot (p < 0.01) in the tubule of exhausted animals. However, the mean esterified cholesterol levels remained unaltered both in the control (0.230 ± 0.032 μM/mg prot) and the exhausted group (0.265 ± 0.027 μM/mg prot).
Figure 2. Total cholesterol (TC), free cholesterol (FC), and cholesterol ester (CE) levels in proximal tubules isolated from sedentary control and five-day exhausted animals (differences from sedentary controls; *p < 0.05, **p < 0.01).
Proximal Tubule Cytoresistance (LDH Release)
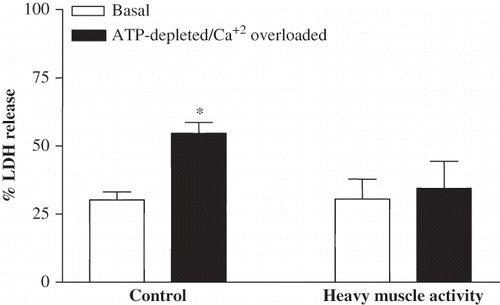
At the basal conditions, after a four-hour incubation at 37°C, LDH release from the isolated proximal tubules into the incubation medium was 30.2 ± 7.07% and 30.59 ± 7.27% in the control and exhausted groups, respectively, whereas LDH release as a response to ATP-depleted/calcium-overloaded conditions, which is used as a second stress model, was statistically different in the tubules of control and exhausted group. LDH release was 54.6 ± 8.2% and 34.41 ± 9.99% (p < 0.001) in the tubule segments of sedentary control and exhausted rats, respectively (see ).
Figure 3. LDH release from proximal tubules isolated from control and five-day exhausted rats. LDH release was determined in the basal and ATP-depleted/Ca+2 overloaded conditions (7.5 μM Antimycin A, 20mM 2-deoxyglucose and 10 μM A23187) and expressed as percentage of total LDH (differences from individual basal conditions; *p < 0.001).
Inorganic Phosphate Level
Total inorganic phosphate of isolated PTS was similar both in sedentary and exercising animals (67.57 ± 8.69 and 69.75 ± 5.40 μg/mg prot, respectively). Mitochondria, isolated from the renal cortex of control and exhausted animals, were used to study the F0/F1 ATPase activity, based on inorganic phosphate release during the 10 min incubation period. Inorganic phosphate releasing effect of F0/F1 ATP synthase in the mitochondria of control and exercised rats was 3.69 ± 0.23 μg.mg prot–1.min–1 and 3.85 ± 0.39 μg.mg prot–1.min–1, respectively.
DISCUSSION
The present study demonstrated that intense physical activity, as confirmed by increased blood lactate concentration and citrate synthase activity in soleus muscle, induces some degree of renal dysfunction associated with proteinuria and glucosuria, without altering MAP, RBF, and GFR. These results are in agreement with the studies reporting on the approving and disapproving effects of vigorous muscle activity on kidney functions.Citation[5–8,10–16,44]
According to Poortmans and Vancalck,Citation[12] proteinuria and glucosuria, seen as a consequence of strenuous physical activity, are indications of impaired glomerular and tubular function, whereas several other authors disagree with the harmful effects of muscular activity because of unaltered kidney functions in trained athletesCitation[45] or exercising animals.Citation[46] In addition, exercise-induced glucosuria and proteinuria are accepted as nonpathological benign processes by Bergstein.Citation[22] In that sense, glucosuria and proteinuria detected in our study may not be regarded as pathological. Because of the lack of a clear consensus on the benefits and risks of acute and chronic exercise on renal functions, the renal cytoresistance theory of Zager et al.Citation[28–32] seems to be useful for the interpretation of our data.
Zager et al.Citation[27–33] believe that when subjected to diverse forms of injury, proximal tubule cells develop adaptive changes as protection against subsequent attacks. Cholesterol loading in the proximal tubule cells is one of these adaptive changes and is accepted as the main criterion for the acquired renal cytoresistance.Citation[27–33] If so, then our result demonstrating the elevated levels of both the total and free cholesterol in the proximal tubule cells of exhausted rats (see ) should indicate evidence of renal insult, and thus heavy muscle activity should be included among the other well-studied insults, such as acute renal failure, ischemia/reperfusion injury, sepsis, endotoxemia, oxidative stress, and urethral obstruction.
It is noteworthy that despite significant cholesterol accumulation in the proximal tubule cells, neither the total nor HDL-LDL cholesterol levels in the plasma were altered significantly in these rats. The mechanistic pathways of cholesterol accumulation in the proximal tubule cells, as response to stress, have been discussed in detail elsewhereCitation[27–33,47] and is not the topic of this study. However, as demonstrated by Zager et al.,Citation[48] in the sepsis-induced mice, the elimination of tubular cytoresistancy in statin-treated exercising rats suggests the involvement of dysregulated cholesterol biosynthesis (unpublished data). A sublethal ATP depletion, which initiates both statin sensitive and resistant cholesterol-loading states in the stressed tubule cells, seems not to be operative in our study. This is indicated by the normal mitochondrial ATP generation in the proximal tubule cells of the exhausted rats. In addition to the existence of normal ATP generation in the tubule segments of exhausted rats, esterified cholesterol levels remained normal, whereas free cholesterol levels were elevated. Contrary to these observations, several authors have reported the predominance of esterified cholesterol accumulation in the tubule cells that are subjected to various forms of stressCitation[33,47] This could be explained by the fact that exercise produces reactive oxygen species (ROS) in the tubule cells,Citation[46,49] which in turn stimulates the PPARγ activity, known to inhibit cholesterol esterification by an ACAT-independent mechanism.Citation[50,51] Thus, the elevation of free cholesterol may be explained by disruption of the fine balance between the exercise-stimulated cholesterol synthesisCitation[52] and ACAT-independent inhibition of cholesterol esterification by ROS stimulated PPARγ. Moreover, as seen in , with the use of an ATP-depleted/Ca+2-overload attack, there was increased release of LDH by the control group of rats as a response to this mitochondrial stress. Unaltered LDH release from the tubule segments of rats exposed to heavy muscle activity indicates membrane resistance in the cholesterol-loaded tubule segments of exercised animals and may represent some adaptive changes as reported by Zager et al.Citation[27–33]
In conclusion, we suggest that the muscle activity model used in this study may be able to stress the proximal tubule cells, thus causing renal cytoresistance. From a clinical point of view, the functional significance of tubular cytoresistancy may gain importance in exercising people subjected to cholesterol-lowering approaches. However, the clinical implications of heavy muscle activity-induced renal cytoresistance remains to be elucidated.
ACKNOWLEDGMENTS
The authors thank Prof. B.U.Yavuzer for her help in the editing of this manuscript. This work was supported by a research grant from Akdeniz University Research Foundation (No: 2005.01.0103.011). The authors report no conflicts of interest.
REFERENCES
- Vatner SF, Higgins CB, White S, Patrick T, Franklin D. The peripheral vascular response to severe exercise in untethered dogs before and after complete heart block. J Clin Invest. 1971;50:1950–1960.
- Millard RW, Higgins CB, Franklin D, Vatner SF. Regulation of the renal circulation during severe exercise in normal dogs and dogs with experimental heart failure. Circ Res. 1972;31:881–888.
- Podhorska-Okołów M, Dziegiel P, Murawska-Ciałowicz E, Exercise-induced apoptosis in renal tubular cells of the rat. Folia Morphol (Warsz). 2004;63:213–216.
- Di Meo S, Venditti P. Mitochondria in exercise-induced oxidative stress. Biol Signals Recept. 2001;10:125–140.
- Momen A, Leuenberger UA, Ray CA, Cha S, Handly B, Sinoway LI. Renal vascular responses to static handgrip: Role of muscle mechanoreflex. Am J Physiol Heart Circ Physiol. 2003;285:H1247–H1253.
- Momen A, Leuenberger UA, Handly B, Sinoway LI. Effect of aging on renal blood flow velocity during static exercise. Am J Physiol Heart Circ Physiol. 2004;287:H735–H740.
- Maeda S, Miyauchi T, Iemitsu M, Endothelin receptor antagonist reverses decreased NO system in the kidney in vivo during exercise. Am J Physiol Endocrinol Metab. 2004;286:E609–E614.
- Middlekauff HR, Nitzsche EU, Nguyen AH, Hoh CK, Gibbs GG. Modulation of renal cortical blood flow during static exercise in humans. Circ Res. 1997;80:62–68.
- Atalay M, Oksala NK, Laaksonen DE, Exercise training modulates heat shock protein response in diabetic rats. J Appl Physiol. 2004;97:605–611.
- Gerth J, Ott U, Fünfstück R, The effects of prolonged physical exercise on renal function, electrolyte balance and muscle cell breakdown. Clin Nephrol. 2002;57:425–431.
- Poortmans JR. Exercise and renal function. Sports Med. 1984;1:125–153.
- Poortmans JR, Vancalck B. Renal glomerular and tubular impairment during strenuous exercise in young women. Eur J Clin Invest. 1978;8:175–178.
- Peters HP, De Vries WR, Vanberge-Henegouwen GP, Akkermans LM. Potential benefits and hazards of physical activity and exercise on the gastrointestinal tract. Gut. 2001;48:435–439.
- Hellebrandt FA, Walters CE, Miller ML. The post exercise suppression of kidney function on man. Am J Physiol. 1936;116:168–173.
- Poortmans JR, Labilloy D. The influence of work intensity on postexercise proteinuria. Eur J Appl Physiol Occup Physiol. 1988;57:260–263.
- Olsen NV, Kanstrup IL, Richalet JP, Hansen JM, Plazen G, Galen FX. Effects of acute hypoxia on renal and endocrine function at rest and during graded exercise in hydrated subjects. J Appl Physiol. 1992;73:2036–2043.
- Donaldson LJ. Sport and exercise: The public health challenge. Br J Sports Med. 2000;34:409–410.
- Khazaeinia T, Ramsey AA, Tam YK. The effects of exercise on the pharmacokinetics of drugs. J Pharm Pharm Sci. 2000;3:292–302; .
- Brown WJ, Burton NW, Rowan PJ. Updating the evidence on physical activity and health in women. Am J Prev Med. 2007;33:404–411.
- Lippi G, Banfi G, Luca Salvagno G, Montagnana M, Franchini M, Cesare Guidi G. Comparison of creatinine-based estimations of glomerular filtration rate in endurance athletes at rest. Clin Chem Lab Med. 2008;46:235–239.
- Zheng H, Li YF, Zucker IH, Patel KP. Exercise training improves renal excretory responses to acute volume expansion in rats with heart failure. Am J Physiol Renal Physiol. 2006;291:F1148–F1156.
- Bergstein JM. A practical approach to proteinuria. Pediatr Nephrol. 1999;13:697–700.
- Maeda S, Iemitsu M, Jesmin S, Miyauchi T. Acute exercise causes an enhancement of tissue renin-angiotensin system in the kidney in rats. Acta Physiol Scand. 2005;185:79–86.
- Boveris A, Navarro A. Systemic and mitochondrial adaptive responses to moderate exercise in rodents. Free Radic Biol Med. 2008;44:224–229.
- Nagashima K, Wu J, Kavouras SA, Mack GW. Increased renal tubular sodium reabsorption during exercise-induced hypervolemia in humans. J Appl Physiol. 2001;91:1229–1236.
- Haskell WL, Lee IM, Pate RR, American College of Sports Medicine; American Heart Association. Physical activity and public health: Updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116:1081–1093.
- Zager RA. Plasma membrane cholesterol: A critical determinant of cellular energetics and tubular resistance to attack. Kidney Int. 2000;58:193–205.
- Zager RA, Burkhart KM, Johnson AC, Sacks BM. Increased proximal tubular cholesterol content: Implications for cell injury and “acquired cytoresistance.”. Kidney Int 1999;56:1788–1797.
- Zager RA, Andoh T, Bennett WM. Renal cholesterol accumulation: A durable response after acute and subacute renal insults. Am J Pathol. 2001;159:743–752.
- Zager RA, Shah VO, Shah HV, Zager PG, Johnson AC, Hanson S. The mevalonate pathway during acute tubular injury: Selected determinants and consequences. Am J Pathol. 2002;161:681–692.
- Zager RA, Johnson AC, Hanson SY. Proximal tubular cholesterol loading after mitochondrial, but not glycolytic, blockade. Am J Physiol Renal Physiol. 2003;285:F1092–F1099.
- Zager RA, Johnson AC, Hanson SY. Renal tubular triglyercide accumulation following endotoxic, toxic, and ischemic injury. Kidney Int. 2005;67:111–121.
- Zager RA, Kalhorn TF. Changes in free and esterified cholesterol: Hallmarks of acute renal tubular injury and acquired cytoresistance. Am J Pathol. 2000;157:1007–1016.
- Ichikawa M, Fujita Y, Ebisawa H, Ozeki T. Effects of long-term, light exercise under restricted feeding on age-related changes in physiological and metabolic variables in male Wistar rats. Mech Ageing Dev. 2000;113:23–35.
- Lowry OH, Rosenbrough NJ, al Farr, Randell RJ. Protein measurement with folin phenol reagent. J Biol Chem. 1951;193:265–275.
- Newman DJ, Price CP. Renal function and nitrogen metabolites. In Burtis CA, Ashwood ER ( eds.). Tietz textbook of clinical chemistry. Philadelphia: WB Saunders Company; 1999:1204–1270.
- Srere PA. Citrate synthase. In Lowenstein JM ( ed.). Methods in enzymology. New York: Academic Press; 1969:3–5.
- Gehrig JJJr, Jamison RL, Baylis C, Troy JL, Brenner BM, Jamison RL. Effect of intermittent feeding on renal hemodynamics in conscious rats. Am J Physiol. 1986;250:F566–F572.
- Agarwal R. Rapid microplate method for PAH estimation. Am J Physiol Renal Physiol. 2002;283:F236–F241.
- Cirrik S, Oner G. Effect of nitric oxide on ammoniagenesis in rats. Nephron Physiol. 2006;102:61–71.
- Vinay P, Gougoux A, Lemieux G. Isolation of a pure suspension of rat proximal tubules. Am J Physiol. 1981;241:F403–F411.
- Weinberg JM, Harding PG, Humes HD. Mitochondrial bioenergetics during the initiation of mercuric chloride-induced renal injury. J Biol Chem. 1982;257:60–67.
- Katewa SD, Katyare SS. A simplified method for inorganic phosphate determination and its application for phosphate analysis in enzyme assays. Anal Biochem. 2003;323:180–187.
- Poortmans JR, Blommaert E, Baptista M, De Broe ME, Nouwen EJ. Evidence of differential renal dysfunctions during exercise in men. Eur J Appl Physiol Occup Physiol. 1997;76:88–91.
- Neumayr G, Pfister R, Hoertnagl H, Mitterbauer G, Prokop W, Joannidis M. Renal function and plasma volume following ultra marathon cycling. Int J Sports Med. 2005;26:2–8.
- Asghar M, George L, Lokhandwala MF. Exercise decreases oxidative stress and inflammation and restores renal dopamine D1 receptor function in old rats. Am J Physiol Renal Physiol. 2007;293:F914–F919.
- Johnson AC, Yabu JM, Hanson S, Shah VO, Zager RA. Experimental glomerulopathy alters renal cortical cholesterol, SR-B1, ABCA1, and HMG CoA reductase expression. Am J Pathol. 2003;162:283–291.
- Zager RA, Johnson AC, Hanson SY. Sepsis syndrome stimulates proximal tubule cholesterol synthesis and suppresses the SR-B1 cholesterol transporter. Kidney Int. 2003;63:123–133.
- Podhorska-Okołów M, Dziegiel P, Dolińska-Krajewska B, Expression of metallothionein in renal tubules of rats exposed to acute and endurance exercise. Folia Histochem Cytobiol. 2006;44:195–200.
- Bilban M, Bach FH, Otterbein SL, Carbon monoxide orchestrates a protective response through PPARgamma. Immunity. 2006;24:601–610.
- Li AC, Binder CJ, Gutierrez A, Differential inhibition of macrophage foam-cell formation and atherosclerosis in mice by PPARalpha, beta/delta, and gamma. J Clin Invest. 2004;114:1564–1576.
- Mahoney DJ, Safdar A, Parise G, Gene expression profiling in human skeletal muscle during recovery from eccentric exercise. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1901–R1910.
