Abstract
Aims. The incidence of complications associated with cerebrovascular diseases in patients who receive hemodialysis for a long-term period is higher than that of other complications. It is known that mortality due to cerebral hemorrhage is two times higher compared to non-dialysis patients. Anti-coagulants used for hemodialysis are essential. Accordingly, in cases in which the cerebral hemorrhage occurred, the selection of anti-coagulants for the prevention of further bleeding poses a great challenge to physicians. The change of hematoma and patient prognosis has a direct relationship. Many ongoing studies are conducted to examine the causative factors causing the increased hematoma and their related prognostic factors. In the current study, we examined the effect of nafamostat mesylate (a serine protease inhibitor) on the change of hematoma compared to heparin in hemodialysis patients. Methods. The current study was conducted in 17 hemodialysis patients who developed a cerebral hemorrhage. These patients were assigned to two groups based on the type of anti-coagulants that they used (i.e., nafamostat mesylate and heparin). Then, the factors affecting the change of hematoma following the onset of cerebral hemorrhage were examined. The prognosis of hematoma was assessed based on brain CT scans, which were performed two weeks after the onset of cerebral hemorrhage in four groups. Following this, groups 1 (the decreased hematoma) and 2 (the decreased delay) were merged to group A (resolving group), and groups 3 (the increased hematoma) and 4 (the death following the aggravation) were merged to group B (the expansion group) for further analysis. Results. There were no significant differences in baseline characteristics between the nafamostat group and the heparin group. A comparison between the resolving group and the expansion group also showed that there were no significant differences in baseline characteristics. In the anti-coagulants and the change of hematoma, however, there were significant differences between the two groups (p = 0.024). A comparison of the change of hematoma between the four groups was also made. This showed that platelet counts and BUN level were significant factors (Platelet; p = 0.042, BUN; p = 0.043 ANOVA with resolving group). Conclusions. Nafamostat mesylate has a similar profile of anti-coagulative activity to heparin. It is assumed, however, that nafamostat has an affirmative effect on the recovery of damaged sites following the onset of cerebral hemorrhage. It is an anti-coagulant that can be safely used for hemodialysis following the onset of cerebral hemorrhage.
INTRODUCTION
Hemodialysis accounts for a majority of renal replacement therapy that is performed in patients with end-stage renal disease. With the increased number of patients who receive hemodialysis for a long-term period, the incidence of complications associated with hemodialysis is also increasing.Citation[1] In dialysis patients, the incidence of cerebral hemorrhage is two times higher than that of normal healthy people. It is well known that cerebral hemorrhage is more prevalently seen than cerebral infarction in dialysis patients.Citation[2] The use of anti-coagulants is unavoidable because dialysis membrane, which is used for hemodialysis, activates the coagulation factors. In patients with end-stage renal disease who developed hemorrhage, hemodialysis is needed to prevent further bleeding due to uremia. Due to this, there is a dilemma that the bleeding recurs. In cases in which the complications greatly affecting the patient prognosis such as cerebral hemorrhage occurred, the further use of anti-coagulants is restricted. In these cases, there will be situations where the efficient dialysis and patient prognosis must be balanced. Nafamostat mesylate (6-amidino-2-naphthyl para-guanidinobenzoate) is a serine protease inhibitor, and has such activities as anti-coagulant effect, anti-fibrinolytic activity, and anti‐platelet actions. Due to a short half life of eight minutes, it has recently been used prevalently concomitantly with heparin as an anti-coagulant for hemodialysis.Citation[3]
We attempted to examine the effect of nafamostat mesylate, which has been reported to cause no further hemorrhage in patients with cerebral hemorrhage that concurrently had bleeding, because it produced a local anti-coagulator activity during the blood coagulation for a short-term period, on the clinical course of cerebral hemorrhage compared to the conventional heparin.
OBJECTIVE AND METHODS
Patients
This study was conducted in patients who were hospitalized for the treatment of hemorrhage in the brain parenchyma due to hypertension of 31 patients who were receiving hemodialysis at Yonsei University Wonju Christian Hospital during a period ranging from January 1, 2004, to August 31, 2007. Of the total cases, those in which the treatment was abandoned due to severe bleeding, those of subarachnoid hemorrhage due to vascular anomaly, those of cerebral hemorrhage due to trauma, those in which there was a bleeding tendency due to other causes, and those in which there was a past history of cerebral lesions including cerebral infarction were all excluded from the current analysis. Of total patients, 17 were included in a follow-up observation. These patients were randomly selected for the use of anti-coagulants. During the follow-up period for two groups (the heparin group and the nafamostat mesylate group), the current study was conducted based on two weeks' brain CT scans and a retrospective review of medical records.
Methods
Study Patients
In 17 subject patients, the age, sex, blood pressure, presence of diabetes mellitus, period of hemodialysis, admission period following the onset of symptoms, Glasgow coma scale, hematoma volume, blood test associated with the hemorrhage, and period of the discontinuation of hemodialysis were checked. To determine whether the hematoma was aggravated, subject patients underwent brain CT two times (at the time of admission and two weeks later). Surgery was performed for general indications (i.e., in cases in which the hematoma volume exceeded 35 ml) in basal ganglia or thalamus and those in which it exceeded 50 ml in the subcortical area. Of total patients, surgery was performed in two cases.
Of total 17 patients, eight used nafamostat mesylate and nine used heparin for the hemodialysis. The prognosis was assessed based on four categories in primary endpoint (viz., decreased cerebral hemorrhage, decreased delay, progression, and death) in both groups. Then, patients were subdivided into two subgroups: the resolving group (combine patients who showed a decreased cerebral hemorrhage with those who had a decreased delay) and the expansion group (combine patients who showed a progression with those who died). Factors affecting the prognosis in two groups in which nafamostat mesylate or heparin was used as an anti-coagulant were analyzed.
Measurement of Hematoma Volume and Its Change
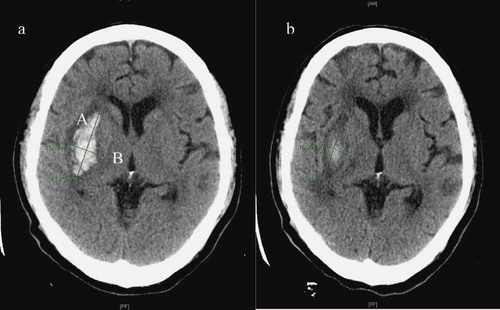
At a two-month interval, a brain CT was performed, and thereby the same level was confirmed. Thus, the areas where the hematoma was maximally observed were compared. To calculate the amount of cerebral hemorrhage, the longest axis was measured using a formula A × B × C/2 and then expressed as a unit of milliliter. Cases in which the length of long axis or the calculated amount of cerebral hemorrhage were decreased on brain CT scans that were performed in the same area (the decreased extent was not defined herein), including the decreased delay, were defined as the resolving group. Cases in which the length of long axis or the calculated amount of cerebral hemorrhage were increased on brain CT scans that were performed in the same area (the increased extent was not defined herein), including death, were defined as the expansion group (see ).
Figure 1. Methods for volume of hematoma and interval change of CT: (a) admission, (b) two weeks later. On the CT slice with the largest area of ICH, the largest diameter (A) of the hematoma was measured by use of the centimeter scale on the CT film. The diameter of the hemorrhage perpendicular to the largest diameter represented the second diameter (B). The height of the hematoma was calculated by multiplying the number of slices involved by the slice thickness, providing the third diameter. The three diameters were multiplied and then divided by 2 (AxBxC/2) to obtain the volume of ICH (this formula was validated by Kwak et al.).
Hemodialysis and the Use of Anti-Coagulants
Hemodialysis was performed using equipment Gambro AK 95® (Gambro, Stockholm, Sweden), and the dialysis membrane used herein was polyamix® (Gambro). Dialysis solution used herein was Bicart® (Gambro). Hemodialysis was performed three times a week at 4 hours/day at a mean blood flow rate of 150–200 mL/min. A loading dose of anti-coagulant (heparin) was 1,000 IU, and this was intravenously infused. A maintenance dose was maximally 200 IU per hour. Nafamostat mesylate was intravenously infused at an hourly dose of 35 mg without a loading dose.
Statistical Analysis
In the current study, statistical analysis was done using SPSS (version 12.0). All of the data were expressed as mean ± SD. Continuous variables were compared using t-test between the heparin and nafamostat groups. Discontinuous variables were compared using a chi-square test. To identify the correlations between the increased hematoma and the related factors, a multiple regression analysis was performed. A value of p < 0.05 was considered statistically significant.
RESULTS
Baseline Characteristics of Total Patients
Total subject patients (n = 17) were composed of ten males and seven females whose mean age was 59.9 ± 9.7 years. Of the underlying diseases, eight had a diabetes mellitus and 17 had hypertension. Mean systolic pressure was 198.7 ± 32.2 mmHg and mean diastolic pressure was 98.6 ± 11.3 mmHg. A blood test showed hemoglobin 10.4 ± 1.5 g/dL, platelet 183.8 ± 72.1 (×1000/L), PT 11.6 ± 1.3 sec, PTT 32.5 ± 4.3 sec, BUN 50.6 ± 24.2 mg/dL, and Cr 7.7 ± 2.7 mg/dL. The period of hemodialysis was 70.6 ± 51.2 months, the amount of cerebral hemorrhage was 20.3 ± 28.9 ml, the Glasgow coma scale (GCS) on admission was 13.9±1.6, the period elapsed until the outpatient visit since the onset of symptoms was 24.8 ± 52.3 hr, and the delayed hemodialysis following the onset of hemorrhage was 67.3±41.6 hr (see ).
Table 1 Epidemiology and characteristics of total patients
The Characteristics Depending on the Anti-Coagulants in Two Groups
A total of 17 patients were assigned to the nafamostat (n = 8) and heparin (n = 9) groups. In these two groups, the following parameters were compared: age, period of hemodialysis, period of outpatient visit following the onset of symptoms, blood pressure, hemoglobin, platelets, PT, aPTT, BUN, Cr, amount of cerebral hemorrhage on brain CT scans, GCS on admission, and period of delayed hemodialysis. There were no significant differences in these parameters between the two groups (see ).
Table 2 Comparison of risk factors between nafamostat and heparin groups
Comparison of Parameters Depends on Change of Hematoma in Two Groups and Four Groups
A total of 17 patients were assigned to the resolving (n = 10) and expansion (n = 7) groups. There were no statistically significant parameters in these two groups. The following parameters were compared: age, period of hemodialysis, period of outpatient visit following the onset of symptoms, blood pressure, hemoglobin, platelets, PT, aPTT, BUN, Cr, amount of cerebral hemorrhage on brain CT scans, GCS on admission, and period of delayed hemodialysis (see ). However, in four groups, there were a difference of platelet counts in the died group (p = 0.042) and BUN in the delayed resolving group (p = 0.043) compared with resolving group in ANOVA (see ).
Table 3 Comparison of risk factors between resolving and expansion groups
Table 4 Comparison of risk factors among four groups about change of hematoma size
Patient Prognosis Depends on Anti-Coagulants in Two Groups
Two weeks later, the changes of hematoma associated with the use of anti-coagulants seen on brain CT scans were evaluated. This showed that the nafamostat group had a significantly better prognosis than the heparin group. To put this another way, patients were assigned to two groups: the resolving group, where patients showed a decreased amount of cerebral hemorrhage or a decreased delay on brain CT scans, and the expansion group, where patients showed an increased amount of cerebral hemorrhage or died. Based on the findings seen on brain CT scans, which were performed two weeks later, it was shown that the nafamostat group had a significantly better prognosis than the heparin group (see ).
Table 5 Chi-square test about CT changes and anticoagulants
A Multiple Logistic Regression Analysis of Risk Factors
To identify the correlations between the findings seen on brain CT scans, performed after two weeks following the onset of cerebral hemorrhage, and the patient prognosis, a multiple logistic regression analysis was performed for such risk factors as the age, period of hemodialysis, period of outpatient visit following the onset of symptoms, blood pressure, hemoglobin, platelets, PT, aPTT, BUN, Cr, amount of cerebral hemorrhage on brain CT scans, coma index on admission, and period of delayed blood dialysis. However, there were no statistically significant results in this series.
DISCUSSION
Hemodialysis accounts for a majority of renal replacement therapy performed in patients with end-stage renal disease. With the advancement of dialysis technology, the number of patients who receive hemodialysis for a long-term period has recently been increasing. According to this, the incidence of complications associated with hemodialysis is also increasing. Of the death causes that have been reported in dialysis patients, excluding cardiovascular diseases, cerebrovascular diseases account for the majority. Of them, the incidence of cerebral hemorrhage is two times higher in dialysis patients than normal healthy people.Citation[2] With the development of hemodialysis techniques, due to the causal relationships with anti-coagulants, which are inevitably used along with the blood transfusion, various new methods have been developed. Of them, nafamostat mesylate has been frequently used in recent years. A serine protease inhibitor, nafamostat mesylate has been known to suppress the coagulation, fibrinolysis, and platelet aggregation. It has therefore been confirmed that nafamostat can be alternatively used to heparin in various clinical areas requiring such anti-coagulant activity.Citation[4,5]
Unfractionated heparin and low-molecular weight heparin are commonly used as anti-coagulants for hemodialysis. Because heparin can cause severe thrombocytopenia and its use is somewhat restricted in patients who concurrently have bleeding, however, thrombin inhibitors such as local citrate and serine protease inhibitors are considered as the second-line measures.Citation[6] The most notable difference in the mechanisms by anti-coagulative effects that occur between nafamostat mesylate and heparin is that the former does not depend on anti-thrombin III. Therefore, rather than acting on several cascades as seen in heparin, nafamostat mesylate directly forms a conjugate with thrombin and thereby blocks the coagulative activity. In addition, it suppresses the activated coagulative factors such as factor XIIa, Xa, plasmin, kallikrein, and complements.Citation[7] It is also involved in the platelet functions; it suppresses the secretion of arachidonic acids including phospholipase A2 and thereby inhibits platelet aggregation. It directly acts on the glycoprotein IIb-IIIa complex and thereby inhibits the activity of various factors that are involved in the blood coagulation pathway.Citation[8] Unlike heparin, which has a sustained systemic effect for four hours, nafamostat mesylate is oxidized in the liver or blood, where it undergoes the structural transformation. Then, it disappears from the blood within several minutes. A sufficient extent of anti-coagulation effects can be obtained when it is intravenously infused at a dose of 40 mg/hr without a loading dose during the process of general hemodialysis. In cases in which the anti-coagulative effect is essential under the circumstances where various types of hemorrhage are induced due to these characteristics, nafamostat mesylate has a similar profile of anti-coagulative effect and produces a lesser extent of side effects compared with heparin. This has been demonstrated in various studies. To date, a substantial number of studies have been conducted to examine whether a serine protease inhibitor can reduce the actual amount of blood loss in cases in which the cardiac open surgery is performed in which the cardiopulmonary bypass is needed. These studies have shown that the actual amount of blood loss was reduced by inhibiting fibrinolysis and preserving platelet counts and their functions.Citation[9]
It is well known that serine protease is involved in the constriction of blood vessels that are distributed in the organs in a shock state due to the bleeding and the suppression of serine protease activity, which plays a crucial role in inhibiting the aggravation of the organ damage.Citation[5] In explaining the constriction of cerebrovascular structures occurring as a result of cerebral hemorrhage, an animal experiment has confirmed that the serine protease pathway including the complements and thrombin was activated in the subarachnoid space in the acute phase following the onset of subarachnoid hemorrhage.Citation[10] Platelet-derived growth factor (PDGF)-BB, which is involved in the vasoconstriction following the onset of subarachnoid hemorrhage, is increased in the basilar artery. It has been demonstrated that a serine protease inhibitor lowers the concentration of PDGF-BB and thereby inhibits the constriction of cerebrovascular structures following the onset of subarachnoid hemorrhage.Citation[11,12] Little is known about the exact mechanism. It is assumed, however, that the decreased effect of a serine protease inhibitor on the hematoma, as seen in the current study, is associated with the suppressing effect on vasoconstriction occurring as the secondary phenomenon in the acute phase following the onset of cerebral bleeding.
Hemorrhage due to uremia occurs with the involvement of various pathophysiologic factors. Little is known about the exact mechanisms. The derangement of platelet aggregation plays a crucial role in the pathophysiology of hemorrhage due to uremia. In cases in which the bleeding occurred, various treatment regimens or methods have been studied to prevent further bleeding. At present, however, such measures as appropriate hemodialysis, erythropoietin, desmopressin, estrogen, and cryoprecipitate have been reported to be effective to some extent. Hemodialysis and the use of erythropoietin are demonstrated methods for the prevention of further bleeding.Citation[7] The mechanisms by which the thrombus is formed during the hemodialysis are based on the activation of platelets due to turbulent flow and shear stress. At a lower rate of blood flow, platelets and thrombin are formed via GPIIb/GPIIIa receptors. To a cellular level, the intrinsic coagulation factors are key players. The concentration of factor XIIa has been known as the determinant for the activation of coagulation pathway.Citation[8] From two aspects, the bleeding due to uremia and the coagulation due to hemodialysis, platelets play a key role. Considering the coagulation factors that are involved in this series, it can therefore be inferred that a serine protease inhibitor is a better anti-coagulant based on the fact that a serine protease inhibitor is advantageous in blocking the major pathway, thereby abolishing the unnecessary effects compared to heparin from several aspects.
In hemodialysis patients, only a few studies have been conducted to examine the predictive factors associated with bleeding (the cerebral hemorrhage in particular) at present. According to a study enrolling 1,064 patients who received blood dialysis for more than three months, however, the cerebrovascular diseases occurred in 9.2% of total patients. Of patients who definitely had a cerebral hemorrhage, 71.4% died within three months following the onset of cerebral hemorrhage.Citation[13] It has been reported that risk factors for developing cerebrovascular diseases include hypertension, left ventricular hypertrophy, and low Kt/V, and they are not associated with albumin or heparin dose in hemodialysis patients.Citation[14,15] To identify the difference in the hematoma in patients with cerebral hemorrhage, a comparison was made between the non-dialysis group and the dialysis group, both of which have no difference in the blood pressure. The location of hematoma was predominantly seen in the lobar shape rather than the basal ganglia or thalamus. In cases in which the intraventricular bleeding was concurrently present, the amount of initial bleeding was significantly greater, and the mortality was approximately two times higher.Citation[16,17] According to a study which was conducted in 41 patients with stroke, 31 had a cerebral hemorrhage. Cerebral hemorrhage was developed approximately ten years earlier in the dialysis group than the non-dialysis group. This study reported that the severity of hypertension, albumin, and cholesterol were involved in the pathophysiology of cerebral hemorrhage.Citation[18] To identify the prognostic factors in association with the increased hematoma following the onset of cerebral hemorrhage, a study was conducted in 24 patients. This showed that the increased hematoma was not associated with the age, sex, blood pressure measured at the time of admission, period elapsed until the hospitalization, or location of hematoma. It has been reported, however, that the coma index on admission, PTT, the size and shape of hematoma, and coagulation factors including fibrin affected the increased hematoma.Citation[19] A study about the prognostic factors was also conducted in 32 patients. In these patients, the activity of daily life (ADL) was compared six months after the onset of cerebral hemorrhage. According to this study, excluding expired patients, the survivors had such involving factors as GCS at the time of admission, age of > 65 years, and blood glucose level of > 200mg/dL. There were no significant differences in other factors.Citation[20] The current study confirmed the traditional risk factors, including age, sex, period of dialysis, period of admission following the onset of symptoms, blood pressure, hemoglobin, platelets, PT, aPTT, BUN, Cr, amount of cerebral hemorrhage seen on CT scans, coma index at the time of admission, and period of delayed dialysis. It was also confirmed that the anti-coagulants had an affirmative effect on the increased hematoma despite a lack of statistical significance. A detailed division of the hematoma change group showed that the decreased platelet counts and the increased BUN had an effect on the progression of hematoma to some extents. It can therefore be inferred that nafamostat mesylate has a greater effect on the derangement of platelet functions and the secondary constriction of blood vessels than heparin in patients with uremia, leading to the affirmative effect on the change of hematoma. Our results indicate that nafamostat mesylate has the additional functions unlike heparin other than anti-coagulative activity. Its role in the vasoconstriction and the change of platelet functions might be more influential.
In conclusion, when the anti-coagulants should be selected for hemodialysis patients who developed cerebral bleeding, nafamostat mesylate is safer and more effective in inhibiting the formation of hematoma than heparin. This deserves further study.
ACKNOWLEDGMENTS
The authors report no external funding.
REFERENCES
- Iseki K, Fukiyama K. Okawa Dialysis Study (OKIDS) Group. Clinical demographics and long-term prognosis after stroke in patients on chronic haemodialysis. The Okinawa Dialysis Study (OKIDS) Group. Nephrol Dial Transplant. Nov2000;15(11):1808–1813.
- Toyoda K, Fujii K, Fujimi S, Kumai Y, Tsuchimochi H, Ibayashi S, Iida M. Stroke in patients on maintenance hemodialysis: A 22-year single-center study. Am J Kidney Dis. Jun2005;45(6):1058–1066.
- Levi M, Cromheecke ME, de Jonge E, Prins MH, de Mol BJ, Briët E, Büller HR. Pharmacological strategies to decrease excessive blood loss in cardiac surgery: A meta-analysis of clinically relevant endpoints. Lancet. Dec 41999;354(9194):1940–1947.
- Zhang Z, Nagata I, Kikuchi H, Xue JH, Sakai N, Sakai H, Yanamoto H. Broad-spectrum and selective serine protease inhibitors prevent expression of platelet-derived growth factor-BB and cerebral vasospasm after subarachnoid hemorrhage: Vasospasm caused by cisternal injection of recombinant platelet-derived growth factor-BB. Stroke. Jul2001;32(7):1665–1672.
- Deitch EA, Shi HP, Lu Q, Feketeova E, Xu DZ. Serine proteases are involved in the pathogenesis of trauma-hemorrhagic shock-induced gut and lung injury. Shock. May2003;19(5):452–456.
- Fischer KG. Essentials of anticoagulation in hemodialysis. Hemodial Int. Apr2007;11(2):178–189.
- Hedges SJ, Dehoney SB, Hooper JS, Amanzadeh J, Busti AJ. Evidence-based treatment recommendations for uremic bleeding. Nat Clin Pract Nephrol. Mar2007;3(3):138–153.
- Weigert AL, Schafer AI. Uremic bleeding: Pathogenesis and therapy. Am J Med Sci. Aug1998;316(2):94–104.
- Murase M, Usui A, Tomita Y, Maeda M, Koyama T, Abe T. Nafamostat mesylate reduces blood loss during open heart surgery. Circulation. Nov1993;88(5 Pt 2):II432–II436.
- Lin CL, Kwan AL, Howng SL. Prognosis of spontaneous intracerebral hemorrhage in hemodialysis patients. Kaohsiung J Med Sci. Aug1999;15(8):484–490.
- Murakami M, Hamasaki T, Kimura S, Maruyama D, Kakita K. Clinical features and management of intracranial hemorrhage in patients undergoing maintenance dialysis therapy. Neurol Med Chir (Tokyo). May2004;44(5):225–232.
- Kawamura M, Fijimoto S, Hisanaga S, Yamamoto Y, Eto T. Incidence, outcome, and risk factors of cerebrovascular events in patients undergoing maintenance hemodialysis. Am J Kidney Dis. Jun1998;31(6):991–996.
- Iseki K, Fukiyama K. Predictors of stroke in patients receiving chronic hemodialysis. Kidney Int. Nov1996;50(5):1672–1675.
- Iseki K, Kinjo K, Kimura Y, Osawa A, Fukiyama K. Evidence for high risk of cerebral hemorrhage in chronic dialysis patients. Kidney Int. Nov1993;44(5):1086–1090.
- Onoyama K, Ibayashi S, Nanishi F, Okuda S, Oh Y, Hirakata H, Nishimura Y, Fujishima M. Cerebral hemorrhage in patients on maintenance hemodialysis. CT analysis of 25 cases. Eur Neurol. 1987;26(3):171–175.
- Miyahara K, Murata H, Abe H. Predictors of intracranial hematoma enlargement in patients undergoing hemodialysis. Neurol Med Chir (Tokyo). Feb2007;47(2):47–51.
- Kazui S, Minematsu K, Yamamoto H, Sawada T, Yamaguchi T. Predisposing factors to enlargement of spontaneous intracerebral hematoma. Stroke. Dec1997;28(12):2370–2375.
- Fujii Y, Tanaka R, Takeuchi S, Koike T, Minakawa T, Sasaki O. Hematoma enlargement in spontaneous intracerebral hemorrhage. J Neurosurg. Jan1994;80(1):51–57.
- Fujii Y, Takeuchi S, Sasaki O, Minakawa T, Tanaka R. Multivariate analysis of predictors of hematoma enlargement in spontaneous intracerebral hemorrhage. Stroke. 1998;29(6):1160–1166 Jun.
- Shin PJ, Han BG, Yoon HJ, Kim JS, Kim MH, Karl EH, Whang K, Choi SO. Clinical features of stroke in patients undergoing maintenance dialysis. Korean J Nephrol. 2000;19:884–890.
