Abstract
Aim: The purpose of this study was to investigate and compare the efficiency of propofol in the reduction of injury induced by free radicals in a rat model of renal ischemia/reperfusion (I/R). Method: Twenty-four Wistar rats were divided into four groups in our study. Rats in the sham group underwent laparotomy and were made to wait for 120 min without ischemia. Rats in the control group were given nothing with ischemia–reperfusion. Rats in the I/R groups were given propofol (25 mg/kg) and 10% intralipid (250 mg/kg) ip, respectively, 15 min before the ischemia for 60 min followed by reperfusion for 60 min. The kidney tissues of the rats were taken under anesthesia at the end of the reperfusion period. Evaluation of biochemical malondialdehyde (MDA), superoxide dismutase, and catalase activities and histopathological analysis were performed with these samples. Results: I/R significantly increased MDA levels (p < 0.05). Histopathological findings of the control group confirmed that there was renal impairment by tubular cell swelling, interstitial edema, medullary congestion, and tubular dilatation. MDA levels were lower in the propofol group compared to control group (p < 0.05). In the propofol group, the level of histopathological scores is significantly decreased than control and intralipid groups in ischemia–reperfusion. Conclusion: Our results demonstrate that I/R injury was significantly reduced in the presence of propofol. The protective effects of propofol may be due to their antioxidant properties. These results may indicate that propofol anesthesia protects against functional, biochemical, and morphological damage better than control in renal I/R injury.
INTRODUCTION
Renal ischemia is a major cause of acute renal failure, initiating a complex and interrelated sequence of events resulting in the injury and death of renal cells.Citation1,Citation2 The prognosis is complicated by the fact that reperfusion causes additional damage (reperfusion injury),Citation3 although essential for the survival of ischemic renal tissue, called ischemia/reperfusion (I/R) of the kidney.
I/R damage can occur in clinical cases such as renovascular surgery, clamping of aorta, shock, trauma, and renal transplantation.Citation4 In renal transplantation process, exposure of the graft, which will be transplanted, to I/R damage in variable levels causes graft function delays and graft losses in postoperative period. Hence in this procedure, it is an indisputable fact that approaches to prevent I/R damage have positive effects on graft functions and graft survey. So before important operations and renal transplantations, it would be wise to find a suitable prophylactic treatment to avoid I/R damage.Citation5
Anesthetic material should be chosen for the patients having risks of kidney failure in addition to kidney diseases and/or operational implementations such as transplantation to protect functions of kidney. However, no ideal anesthetic material has been developed to protect the functions of kidney, yet.Citation6 Propofol has been reported to have a protective effect against I/R injury in several organs such as heart,Citation7 brain,Citation8 and lower limbs.Citation9 The aim of this study is to examine the possible effects of propofol against the damage caused by I/R in renal tissue.
METHOD
Animals
The experimental protocol used in this study was approved by the Animal Ethics Review Committee of the Faculty of Medicine, University of Kahramanmaras, and adhered to National Institutes of Health guidelines for the use of experimental animals. Animals were housed in individual cages in a temperature-controlled room with alternating 12-h light–dark cycles and acclimatized for a week before the study. Food was removed 8 h prior to the study, but all animals were allowed free access to water and rat chow diet.
Experimental design
Animals were anesthetized with intramuscular injections of 50 mg/kg ketamine hydrochloride (Ketalar, Eczacıbaşı, Turkey). The abdominal region was shaved and sterilized with povidone iodine solution. A midline incision was made and the abdominal viscera were retracted to the right side. The left renal hilus was dissected, the renal vascular pedicle was occluded using a microvascular clamp (REDA Instrument, 13111-06, Tuttlingen, Germany), and the intestine was replaced into the abdominal cavity. At the end of 60-min ischemic period, 60 min of reperfusion was established by removal of the clamp, and left nephrectomy was performed. Animals were randomly divided into four groups, each consisting of six animals. Group 1 (sham group, n = 6) rats were subjected to identical surgical procedures described above except for renal I/R. Group 2 (I/R group, n = 6) rats received 60 min of left renal ischemia followed by 60 min of reperfusion. Group 3 (I/R + propofol group, n = 6) animals were administered propofol (25 mg/kg, ip; Propofol 1% Fresenius, Fresenius Kabi AB, Uppsala, Sweden) 15 min before the reperfusion phase. Group 4 (I/R + intralipid group, n = 6) animals were administered intralipid (250 mg/kg, ip; intralipid 10%, 500 mL Fresenius, Fresenius Kabi AB, Uppsala/Sweden) 15 min before the reperfusion phase. Routinely all the rats' renal vascular pedicle was occluded using a microvascular clamp, at the end of 60 min ischemic period, 60 min reperfusion was established by removal of the clamp, and left nephrectomy was performed. At the end of the reperfusion period, the sample tissues were harvested. Half of the tissues were used for tissue biochemical analysis and the other half for histopathological evaluation.
Renal histology
The kidneys fixed in a 10% neutral buffered formalin solution were embedded in paraffin and were used for histopathological examination.Citation10 Five-micrometer thick sections were cut, deparaffinized, hydrated, and stained with hematoxylin and eosin. The renal sections were examined in blind fashion for tubular cell swelling, tubular dilatation, hyaline cast, interstitial edema, medullary congestion, and moderate to severe epithelium necrosis in all treatments. A minimum of 10 fields for each kidney slide were examined and assigned for severity of changes using scores on a scale of none (−), mild (+), moderate (++), and severe (+++) damage ().
TABLE 1. Effect of propofol treatment on morphological changes as assessed by histopathological examination of kidneys of the rats exposed to renal I/R
Antioxidant study
To determine tissue antioxidant levels, 1 × 1 cm2 tissue samples were taken from the left lateral part of the incision line on the abdominal wall. The samples were preserved in a deep freezer until examination. The tissues were homogenized with three volumes of ice-cold 1.15% KCl. The activities of antioxidant enzymes and the levels of lipid peroxidation were measured in the supernatant obtained from centrifugation at 14,000 rpm. Superoxide dismutase (SOD) activity was measured according to the method described by Fridovich.Citation11 Catalase (CAT) activities were determined by measuring the decrease in hydrogen peroxide concentration at 230 nm according to the method of Beutler.Citation12 Lipid peroxidation level in the tissue samples was expressed in malondialdehyde (MDA) and measured according to the procedure of Ohkawa et al.Citation13 Protein concentration was determined according to the method of Lowry.Citation14
Statistical analysis
All variables were expressed as mean ± standard deviation. Differences between groups were evaluated by Kruskal–Wallis variance analysis followed by a post hoc Mann–Whitney U-test. p-Values <0.05 were considered statistically significant. All data were entered into and processed by SPSS 9.05 for Windows statistical package.
RESULTS
I/R significantly increased MDA levels (p < 0.05) and decreased CAT activities (p < 0.05), and did not change SOD levels (p > 0.05). Histopathological findings of the control group confirmed that there was renal impairment by tubular cell swelling, interstitial edema, medullary congestion, and tubular dilatation.
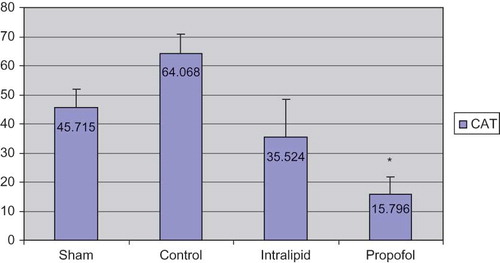
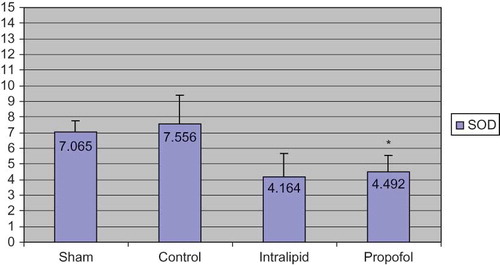
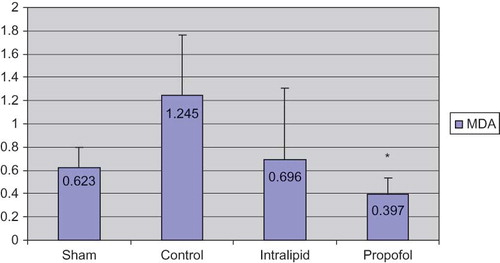
When the antioxidant levels of half of the renal tissues were evaluated the following results were obtained: Group 1 (sham): 0.623 ± 0.177; Group 2 (control): 1.245 ± 0.518; Group 3 (intralipid): 0.696 ± 0.614; Group 4 (propofol): 0.397 ± 0.134 (). The MDA values of propofol group were significantly lower than the control group (p < 0.05) and levels in intralipid group were higher than the propofol group. In propofol group, the levels of histopathological scores were significantly decreased than control groups in ischemia–reperfusion. The values of CAT were as follows: Group 1: 45.715 ± 6.168; Group 2: 64.068 ± 6.881; Group 3: 35.524 ± 12.911; Group 4: 15.796 ± 6.044 (). CAT levels were high in control and intralipid groups; the differences between propofol group and control group were statistically significant. If the SOD levels were taken into account, the values were as follows: Group 1: 7.065 ± 0.687; Group 2: 7.556 ± 1.820; Group 3: 4.164 ± 1.504; Group 4: 4.492 ± 1.080 (). The values of SOD in the control group were higher than the propofol group (statistically significant). It was found that the results of the intralipid group for SOD were higher compared to propofol group, but it did not reach statistical significance when these groups were compared.
FIGURE 1. MDA levels (nmol/mg protein).
DISCUSSION
The most important reason of acute kidney failure is kidney ischemia, which causes damage to and death of kidney cells. In kidney transplantation, I/R damage is one of the most important factors. Reperfusion damage is the chain of events related to free oxygen radicals produced during tissue ischemia and reperfusion.Citation15 The kidney I/R damage which is a critical clinical problem has become the subject of many clinical and experimental studies with the development of transplantation surgery. In kidney I/R damage, there are some comparative studies between propofol and other anesthetic agents.Citation16,Citation17 Basu et al. detected that propofol decreases oxidative stress and inflammatory response in patients who had kidney transplantation.Citation18
As oxygen radicals are short-lived and the kidney tissue has a complex structure, it is hard to detect oxygen radicals. It is detected indirectly by measuring the lipid peroxidation originating from free oxygen radicals. One of the products produced by lipid peroxidation is MDA.Citation19 So, in our research, we studied MDA levels in the kidney tissues to show the damage and compare the material effects in decreasing the damage. We found that the highest MDA rate is in control group when we examined the MDA levels we established. We determined that MDA levels of propofol group were low compared to control group (p < 0.05). Consequently, according to our results, propofol seems to have some advantages for the prevention of oxidative stress and peritoneal adhesions in comparison with other groups.
During metabolism, there are enzymatic organisms which are against the harmful effects of free oxygen radicals. Measuring these enzyme levels gives us indirect information about the free radical meditative damage. We examined SOD and CAT levels in kidney tissue. Examining the SOD levels, we found that the lowest level is in the propofol group and the highest level is in the control group. Thus, our findings support propofol-scavenging properties. Moreover, anesthesia conducted with propofol reduced oxidative stress and enhanced antioxidant defense mechanisms expressed by larger concentrations of free radical scavengers.
In the histopathological analysis of kidney tissue of propofol group significant recovery was observed. Considering the increase in average histological scores, it was also found to be statistically low compared to control group. The anesthetic material propofol that has high-lipid resolution is often used in induction and prosecution of anesthesia and in sedation of patients bound to mechanical ventilators in intensive care unit. The positive effects of many organs such as heart, lungs, brain, liver, and testis were observed. Propofol may limit the oxidative damage in various tissues including kidney. Besides, the effect of propofol on the kidney I/R damage has been stated in very few studies. In the study of Wang et al.Citation20 propofol was found to decrease significantly the kidney ischemia–reperfusion damage and renal dysfunction in rats. Furthermore, they showed that these protective effects are via heme oxygenase-1 induction. Yagmurdur et al. showed in their studies that propofol hypoperfusion–reperfusion condition related to lipid peroxidation inhibition and induction doses of propofol may be advantageous in suspensions of metabolites and free oxygen radicals.Citation21 In their study examining tissue antioxidant capacities during propofol anesthesia, Runzer et al.Citation22 detected that MDA levels decrease significantly when high doses of propofol is mixed with halothane. Nonetheless in the same study, it was detected that the protective effect of propofol is significant in all tissues and has a significant protective effect primarily in liver and then kidney, heart, and lungs. When adding propofol in high and low doses, much positive results were observed in high doses. They explained the different responses of tissues as each tissue has different lipid peroxidation sensitivity.
The propofol forms used in clinics are preparations prepared as 10% lipid emulsion. In this study of how those components affect the I/R damage, we also used intralipid solution containing 10% lipid contents as another group. In his study, Szekely mentions about intralipid explosion reaction redoubling effect by neutrophils.Citation23 Mathy-Hartert, on the other hand, compared propofol and intralipid in his study of the effects of free oxygen radicals. It is stated that intralipid has a low level effect.Citation24 In Runzer's study, it is confirmed that intralipid has a low level of antioxidant effect. In combinations of intralipid and propofol, however, they stated that in low concentrations, this effect is more evident.Citation22 Our diagnoses are supported by the results of those studies. In our study, we confirmed that intralipid in rats may reduce kidney I/R damage in a low level; however, propofol lowers kidney I/R damage in a significant level.
The most important finding in this study was that the administration of propofol after ischemia prevented the marked reperfusion injury that was detected in the control group. Finally, our data indicate that exposure to propofol reduces lipid peroxidation and enhances antioxidant defenses. Further experiments are necessary to elucidate the mechanisms of action of propofol in a condition of bacterial peritonitis indication.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Thadhani R, Pascual M, Bonventre JV. Acute renal failure. N Engl J Med. 1996;334(22):1448–1460.
- Lieberthal W, Levine JS. Mechanisms of apoptosis and its potential role in renal tubular epithelial cell injury. Am J Physiol. 1996;271:477–488.
- Weight SC, Bell PR, Nicholson ML. Renal ischaemia – reperfusion injury. Br J Surg. 1996;83(2):162–170.
- Alatas O, Sahin A, Colak O, Beneficial effects of allopurinol on glutathione levels and glutathione peroxidase activity in rat ischemic acute renal failure. J Int Med Res. 1996;24(1):33–39.
- Pratt JR, Jones ME, Dong J, Nontransgenic hyperexpression of a complement regulator in donor kidney modulates transplant ischemia/reperfusion damage, acute rejection, and chronic nephropathy. Am J Pathol. 2003;163(4):1457–1465.
- Rusafa Neto E, Vianna PT, Viero RM, Influence of S(+)-ketamine analgesia in renal intraoperative ischemia: Histological study in rats. Acta Cir Bras. 2006;21(4):242–246.
- Kokita N, Hara A, Abiko Y, Arakawa J, Hashizume H, Namiki A. Propofol improves functional and metabolic recovery in ischemic reperfused isolated rat hearts. Anesth Analg. 1998;86:252–258.
- De La Cruz JP, Villalobos MA, Sedeno G, De La Cuesta FS. Effect of propofol on oxidative stress in an in vitro model of anoxia-reoxygenation in the rat brain. Brain Res. 1998;800:136–144.
- Aldemir O, Celebi H, Cevik C, Duzgun E. The effects of propofol or halothane on free radical production after tourniquet induced ischaemia-reperfusion injury during knee arthroplasty. Acta Anaesthesiol Scand. 2001;45:1221–1225.
- Paller MS, Neuman TV. Reactive oxygen species and rat renal epithelial cells during hypoxia and regeneration. Kidney Int. 1991;40:1041–1049
- Fridovich I. Superoxide radical: An endogenous toxicant. Annu Rev Pharmacol Toxicol. 1983;23:239–257.
- Beutler E. Red Cell Metabolism. 2nd ed. New York: Grune & Stratton; 1975.
- Ohkawa H, Ohishi N, Tagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275.
- Hourmant M, Vasse N, le Mauff B, Soulillou JP. The role of adhesion molecules in ischaemia-reperfusion injury of renal transplants. Nephrol Dial Transplant. 1997;12(12):2485–2487.
- Lee HT, Chen SW, Doetschman TC, Deng C, D‘Agati VD, Kim M. Sevoflurane protects against renal ischemia and reperfusion injury in mice via the transforming growth factor-beta1 pathway. Am J Physiol Renal Physiol. 2008;295(1):128–136. (Abstract).
- Sánchez-Conde P, Rodríguez-López JM, Nicolás JL, The comparative abilities of propofol and sevoflurane to modulate inflammation and oxidative stress in the kidney after aortic cross-clamping. Anesth Analg. 2008;106(2):371–378.
- Basu S, Meisert I, Eggensperger E, Krieger E, Krenn CG. Time course and attenuation of ischaemia-reperfusion induced oxidative injury by propofol in human renal transplantation. Redox Rep. 2007;12(4):195–202.
- Paller MS, Hoidal JR, Ferris TF. Oxygen free radicals in ischemic acute renal failure in the rat. J Clin Invest. 1984;74(4):1156–1164.
- Wang HH, Zhou HY, Chen CC, Zhang XL, Cheng G. Propofol attenuation of renal ischemia/reperfusion injury involves heme oxygenase-1. Acta Pharmacol Sin. 2007;28(8):1175–1180.
- Yagmurdur H, Cakan T, Bayrak A, The effects of etomidate, thiopental, and propofol in induction on hypoperfusion-reperfusion phenomenon during laparoscopic cholecystectomy. Acta Anaesthesiol Scand. 2004;48(6):772–777.
- Runzer TD, Ansley DM, Godin DV, Chambers GK. Tissue antioxidant capacity during anesthesia: Propofol enhances in vivo red cell and tissue antioxidant capacity in a rat model. Anesth Analg. 2002;94(1):89–93
- Szekely A, Heindl B, Zahler S, Conzen PF, Becker BF. Nonuniform behavior of intravenous anesthetics on postischemic adhesion of neutrophils in the guinea pig heart. Anesth Analg. 2000;90(6):1293–1300.
- Mathy-Hartert M, Deby-Dupont G, Hans P, Deby C, Lamy M. Protective activity of propofol, Diprivan and intralipid against active oxygen species. Mediators Inflamm. 1998;7(5):327–333.