Abstract
Background: Co-administration of diltiazem and cyclosporine A (CsA) in kidney transplant recipients shows improvement of renal transplantation outcomes. Methods: We respectively analyzed 1531 kidney transplant recipients treated by different immunosuppressive therapy schemes from 1986 to 2003. They were divided into three groups depending on their immunosuppressive therapy schemes: control group with a standard triple therapy without use of diltiazem; study group I with the combination of diltiazem and the standard triple therapy but slightly low CsA; study group II with combination of diltiazem and a modified standard triple therapy but lower CsA. The CsA blood concentrations, posttransplant complications, and long-term survival in the three groups were compared. Results: The results showed that the patient and allograft survival in the study group II was 69.9 and 65.1%, respectively, significantly higher than that in the control group (50.7 and 47.6%). Occurrence of hepatotoxicity and nephrotoxicity episodes was higher in the control group than those in the study group I and the study group II. The incidence of acute rejection in the control group was 30.3% (23/76), similar to 28.0% (184/657) in study group I, but statistically significantly higher than 7.6% (61/798) in the study group II. Conclusion: Combination of diltiazem and CsA in the kidney allograft recipients tends to reduce the CsA oral dosage, improve patient survival, and decrease the occurrence of hepatotoxicity and nephrotoxicity.
INTRODUCTION
The introduction of cyclosporine A (CsA), an immunosuppressive agent, significantly improves the solid kidney transplantation outcomes.Citation1 However, higher cost for long-term immunosuppressive therapy has been a heavy financial burden to the kidney allograft recipients especially in developing such as China. In addition, cyclosporine is reported to be associated with hypertension,Citation2 and long-term side effect such as nephrotoxicity is also a major concern.Citation3 Co-administration of cyclosporine and diltiazem, a calcium channel blocker, results in increased cyclosporine blood levels that subsequently require a small dose of CsA to be used while maintaining a same CsA blood level.Citation4–6 This combination has also been approved to reduce the incidence of both graft failure and graft rejection episodes.Citation7 As an anti-hypertension agent, diltiazem probably helps to reduce high blood pressure caused by CsA. Randomized trials of co-administration of cyclosporine and diltiazem in transplant patients show the significant reduction of daily dose of cyclosporine but this reduction has not resulted in any observed side effect including patient survival, graft rejection, or other complications.Citation8,Citation9 However, these studies are designed generally with small sample size, short-term follow-up, and most in developed countries. Long-term outcome of this combination with a large sample size has rarely been observed in developing countries. The objectives of this study were to: (i) examine the CsA blood levels in the kidney transplant patients receiving both CsA and diltiazem, and in the patients receiving CsA but no diltiazem; (ii) investigate the long-term allograft and patient survival in the patients with co-treatment of diltiazem and CsA and in the transplant patients with CsA alone; (iii) compare the occurrence rates of acute rejection and nephrotoxicity and hepatotoxicity episodes in the transplant patients with co-administration and the patients with CsA alone.
METHODS
Subjects
Primary kidney transplant recipients who were performed kidney transplantation and administered CsA at the First Affiliated Hospital of Xi'an Jiaotong University, China, between 1986 and 2003 were recruited in this study. The demographic data are presented in . Inclusion criteria for patients were (i) a stable physical condition after kidney transplantation; (ii) patients with continuing CsA therapy for at least 12 months; and (iii) patients with at least 1-year follow-up and complete data of CsA dose and blood levels. Patients who died or experienced graft failure within 1 month after kidney transplantation and received other immunosuppressive therapy agents or intermittent administration of diltiazem were excluded from this study.
TABLE 1. Demographics of kidney transplant recipients
Study design
The enrolled patients were allocated into three groups (study group I, study group II, and control group) depending on their immunosuppressive therapy schemes. Patients in the study group I orally received diltiazem (60 mg p.o. tid) plus the standard therapy that consisted of reduced CsA (6.5 mg/kg/d for initial dose and gradually decreased to 3–4 mg/kg/d), azathioprine (AZA) (50–75 mg/d), and prednisolone (40 mg/d for initial and 15–20 mg/d for maintaining dose). Patients in the control group orally administrated with the standard therapy (CsA at 8.0–9.0 mg/kg/d for initial dose and gradually decreased to 3–4 mg/kg/d) as the study group I but no diltiazem. Finally, 657 cases in the study group I and 76 cases in the control group with complete data collection were used for analysis in this study. In the study group II, 798 patients orally received diltiazem (same as study group I, 60 mg p.o. tid) concurrently with a slightly different standard therapy that consisted of CsA but commenced at 4.5–5.0 mg/kg/d for initial dose and maintained at 2.0–3.0 mg/kg/d (low dose), prednisolone, and a dose of mycophenolate mofetil (MMF) (for replacement of AZA). CsA blood levels were examined for at least 1-year posttransplantation, and blood CsA was tested by a monoclonal antibody-based fluorescence polarization immunoassay. HLA-A and HLA-B typing was determined by a lymphocytotoxicity assay, and HLA-DR typing was determined by a polymerase chain reaction–sequence-specific primers (PCR–SSP) method. ABO blood typing between donors and recipients was all matched. The pretransplantation data, such as HLA mismatch, panel reactive antibody levels, donor's information, and posttransplantation data including survival, immunosuppressive agent application, and health status in all kidney allograft recipients were entered into a kidney transplantation follow-up database system, which we regularly collect, update, and maintain the kidney transplant patients' laboratory, clinical, and epidemiological information.
Definitions
Nephrotoxicity associated with CsA was diagnosed when presenting an elevated serum creatinine that could not be explained by other causes. Hepatotoxicity was diagnosed when an abnormal liver function appeared with negative virus test, and the recovery of liver function in association with the reduction of CsA dosage was observed. In early years, acute rejection episodes in kidney allograft recipients were diagnosed by the clinical symptoms. In recent years, the suspected acute rejection episodes were biopsied and graded according to the 1997 Banff classification.Citation10 Infection was defined when patients were diagnosed as pneumonia, urinary infection, positive for human cytomegalovirus (HCMV) DNA and Epstein–Barr virus (EBV) DNA by PCR assays, seroconvert for CMV IgG and IgM by enzyme-linked immunoassays (ELISA), and/or immunofluorescence antigenemia for CMV pp65 antigen.
Data analysis
One-way ANOVA using SPSS software (Chicago, IL, USA) was performed to compare serum CsA levels between the two study groups and the control group. The 1-, 3-, and 5-year allograft and patient survival rate in three groups were evaluated using life table analysis by Fisher's test. Comparison of long-term side effects, the occurrence rates of nephrotoxicity, hepatotoxicity, acute rejection, and infection episodes among three groups were determined by Chi-square analysis.
RESULTS
During 1986–2003, approximately 1653 kidney transplant patients were performed in the First Hospital of Xi'an Jiaotong University. Based on the inclusion and exclusion criteria for the patient enrollment, data from 1531 cases were retrospectively analyzed. They were allocated to control group, study group I, or study group II depending on their immunosuppressive therapy schemes. Out of these cases, 1526 patients received allograft from cadaveric donors and only 5 from living donors. The details of demographic data are summarized in . In general, the baseline characteristics have no statistically significant difference among patients in the study group I, the study group II, and the control group.
CsA oral dosage and serum levels in three groups
Of all the recruited patients, CsA daily doses per kilogram body of weight after transplantation were followed up regularly and entered into the data system. The CsA serum levels were tested at the time of hospitalization (usually within 1-month posttransplantation) and during the follow-up visits (2–3, 4–6, 9–12 months, and a year later) after the patients were discharged from the hospital. shows the average CsA dose and CsA serum levels in the three groups at different intervals of posttransplantation. Although the average CsA oral doses in the study group I were significantly lower (p < 0.05) than those in the control group at all intervals over 1 year, the average CsA serum levels in the study group I were similar to those in the control group (p > 0.05) at any individual time. Comparison of the average dose at 9–12 months interval indicates that the oral CsA dose was reduced by approximately 0.42–0.63 mg/kg/d in the study group I, a 10–15% reduction compared with that in the control group (data not shown). In the study group II, the oral CsA dose was reduced by an average of 0.93 mg/kg/day, a 22% reduction than that in the control group during 9–12 months, which is statistically significant different than that in the control group and in the study group I. In addition, the CsA serum levels in the study group II were significantly lower (p < 0.01) than those in the control group and the study group I at all intervals to 12 months. When the CsA cost was compared, the costs in the study group II were almost reduced by 40% than those in the control group (data not shown) at 9–12 months posttransplantation.
FIGURE 1. Average oral CsA dose (mg/kg/d) and CsA serum levels (mg/L) at <1, 2–3, 4–6, 9–12, and >12 months posttransplantation in the study group I with a combination of diltiazem and a standard triple therapy [slightly reduced CsA (6.0 mg/kg/d), AZA, and prednisolone], in the study group II with a combination of diltiazem and a slightly different triple [reduced CsA (4.5–5.0 mg/kg/d), prednisolone, and mycophenelate mofetil] therapy, and in the control group with a standard triple therapy [normal CsA (8.0–9.0 mg/kg/d), AZA, and prednisolone].
![FIGURE 1. Average oral CsA dose (mg/kg/d) and CsA serum levels (mg/L) at <1, 2–3, 4–6, 9–12, and >12 months posttransplantation in the study group I with a combination of diltiazem and a standard triple therapy [slightly reduced CsA (6.0 mg/kg/d), AZA, and prednisolone], in the study group II with a combination of diltiazem and a slightly different triple [reduced CsA (4.5–5.0 mg/kg/d), prednisolone, and mycophenelate mofetil] therapy, and in the control group with a standard triple therapy [normal CsA (8.0–9.0 mg/kg/d), AZA, and prednisolone].](/cms/asset/cf5d4f62-9ac7-4e3f-a7b3-5543e086c4db/irnf_a_461136_f0001_b.gif)
The patient and allograft survival rates in different treatment groups
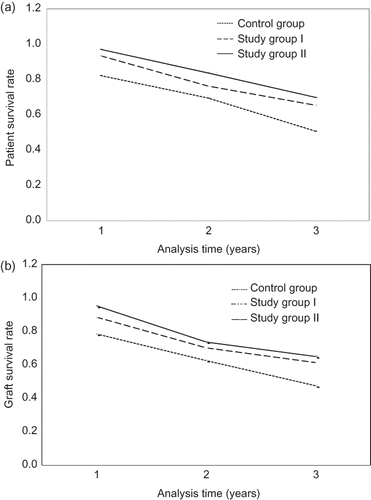
In the study group I, at 1 year, the average patient and allograft survival was 93.5 and 88.6%, respectively. This was reduced to 76.4 and 70.2% at 3 years, and 65.5 and 61.4% at 5 years. Similarly, in the study group II, the average patient and allograft survival rate at 1, 3, and 5 years was 95.4 and 93.6%, 83.8 and 73.8%, and 69.9 and 65.1%, respectively. In contrast to the study group, the average patient and allograft survival at 1, 3, and 5 years in the control group was 82.3 and 78.5%, 69.5 and 62.7%, and 50.7 and 47.6%, respectively. reveals that the patient and the allograft survival patterns over 5 years are very similar. The average survival in the study group II was statistically significantly higher (p < 0.05) than those in the study group I over 5 years. Interestingly, the average individual survival in either study group I or study group II was significantly higher (p < 0.05) than those in the corresponding control group after the first year follow-up and thereafter. We further followed up the detailed causes of allograft failure in each group. Chronic allograft nephropathy (CAN) was the major cause of allograft failure, accounting for 71.7, 75.5, and 78.6% of the total allograft failure cases in the study group II, the study group I, and the control group, respectively. The second cause of allograft failure was acute rejection that occurred 18.7, 17.9, and 14.3% in the study group II, the study group I, and the control group, respectively.
FIGURE 2. Patient (a) and graft (b) survival rates of kidney allograft recipients over 5-year follow-up. (a) shows the patient survival rates at 1-, 3-, and 5-year follow-up in the study group I, the study group II, and the control group; similarly (b) shows the corresponding graft survival rates at 1-, 3-, and 5-year follow-up in the three groups.
Posttransplant complications
Fourteen nephrotoxicity episodes (18.4%) occurred in the control group, which differs from 71 episodes (10.8%) in the study group I and 53 episodes (6.6%) in the study group II (). Similar as nephrotoxicity episodes, hepatotoxicity occurred 28.9% in the control group, significantly higher than that in the study group I (19.9%) or the study group II (12.3%). Reduced nephrotoxicity and hepatotoxicity episodes in the study groups are probably due to reduced use of CsA and additional application of diltiazem that might decrease the hepatic and kidney toxicity and simultaneously protect their functions. Interestingly, infection rates including CMV, pneumonia, urinary tract infections (UTIs), and EBV infections in the control group were significantly higher (p < 0.05) or no different than those in the study groups (p > 0.05), indicating that immunosuppressive therapy might increase infection opportunities.
TABLE 2. The occurrence rates of complications posttransplant in three groups
The incidence of acute rejection, diagnosed by clinical symptoms in early years and by allograft biopsy in recent years, in the control group was 30.3% (23/76), similar to 28.0% (184/657) in study group I, but statistically significantly higher than 7.6% (61/798) in the study group II.
DISCUSSION
After kidney transplantation, the kidney allograft recipients need to be accordance with a long-term immunosuppressive therapy. Among all the immunosuppressive agents, CsA has been proved to be one of the most effective agents to protect transplant kidneys posttransplantation. However, its application still exhibits problems. The primary disadvantage is expensive, which causes a severely economic burden to the kidney allograft recipients. Due to the high cost of CsA, some patients have to stop or reduce CsA daily doses posttransplantation, resulting in frequent episodes of kidney rejection and impacting the long-term graft survival and the life quality. This phenomenon is more common in the developing countries. Therefore, it is critical to guarantee a good outcome of transplantation and simultaneously reduce the CsA doses. In 1986, GrinoCitation11 firstly reported that co-administration of diltiazem and CsA could inhibit the metabolism of CsA; as a result, this combination caused increased CsA levels in blood and a reduced CsA dosage needed for maintaining normal kidney function. Since that time, several studies have observed the outcome of diltiazem co-administration but they are all designed with short-term follow-up.Citation4,Citation12 Studies determining the long-term outcome of co-administration in kidney transplant patients, especially studies in developing countries, are sparse. We started diltiazem co-treatment with CsA in kidney transplant patients in 1986. One thousand five hundred and thirty-one cases of kidney allograft recipients had been followed up for at least 1 year for their CsA serum levels and approximately 5 years for their outcomes of transplantation.
In this study, although the CsA doses in the study group I, which diltiazem was supplemented, were reduced by approximately 10–15% than those in the control group that diltiazem was not used, the CsA serum levels in the study group I were similar to those in the control group at all intervals during the first month and up to 12 months posttransplantation. In the study group II that diltiazem was also applied, the CsA doses were further reduced by approximately 40% than those in the control group; the CsA serum levels were also significantly lower than those in the control group at all follow-up time. Because CsA was proved to be associated with long-term nephrotoxicity,Citation3 and CsA doses were significantly reduced in the study groups, therefore, reduction of CsA doses may be the major cause of fewer episodes of hepatotoxicity and nephrotoxicity in the study group II than those in the control group (). In addition, diltiazem supplement in the study groups is also likely associated with reduction of hepatotoxicity and nephrotoxicity episodes because diltiazem causes less toxicity to livers and kidneys, therefore leading to the protection of kidney function. Moreover, reduced CsA dosage applied in the study group II did not result in the increase of the acute rejection episodes, which indicates that reduced CsA dosage with concurrent use of diltiazem is safe and efficacious for the allograft recipients. In this study, the incidence of acute rejection was 18.4% in control group, 10.8% in study group I, and 6.6% in study group II. Reduced acute rejection episodes in study group II tend to be associated with the application of MMF that proved superior to AZA as a posttransplant immunosuppressant and MMF has less toxicity to allograft than AZA.Citation13,Citation14
It suggests that CsA induces nephrotoxicity,Citation3 which likely further results in CAN which may cause graft failure or even death. Therefore, CAN was considered an important factor impacting the long-term survival of allograft and patients.Citation15,Citation16 Because CAN is a gradual procedure, kidney function at early stage tends to be normal but gradually deteriorates. Study indicates that reduced dose of CsA obviously decreased the occurrence of CAN.Citation17 In this study, doses and levels of CsA in study group I and study group II were lower than those in control group in the first year. As a result, reduced doses of CsA may decrease occurrence of CAN, graft failure, and death, subsequently leading to improvement of 3- and 5-year patient/graft survival. In addition, because of the use of diltiazem in study groups that likely protect kidney function, this likely further improve the patient and allograft survival rates in the study group II and the study group I. This conclusion was not supported by a recent study in Thailand, which did not show an improvement of allograft survival between the diltiazem and the non-diltiazem groups.Citation6 Although the survival rates were improved in the study groups than those in the control group, however, the infection rates in the three groups were relatively higher, and no significant difference was observed between one to the other group, indicating that immunosuppressive therapy may increase the opportunities of infection no matter what immunosuppressive therapy scheme was used. Unfortunately, we did not follow up the immunosuppressive agent use status of all the patients over 1 year after their discharging from the hospital, but the limited data indicate that most of the patients likely keep the immunosuppressive therapy pattern similar to when they started the therapy, and a small number of patients either stop using immunosuppressive therapy due to financial reason or slightly change the dose of the co-administration formula. This disadvantage seems unlikely to impact the overall results and conclusions from this study.
These results suggest that concurrent use of diltiazem can significantly raise CsA blood levels and reduce the required CsA dosage in kidney transplant patients. Due to the reduction of CsA doses, the patients' financial burden is much alleviated, and it further guarantees the outcome of transplantation and offers an effective method for the long-term CsA application. McCauleyCitation18 reported that CsA daily dosage could reduce 30–50% after co-administrating diltiazem, and it can save approximately $3000 per patient annually. Our study reveals that 10–15% CsA doses could be reduced after co-treatment of diltiazem with CsA + Aza + Pred (study group I) which can save nearly ¥5000–6000 ($690–830) per patient every year (data not shown). In the CsA + MMF + Pred group (study group II), 25–40% CsA doses were reduced after administration of diltiazem, and this results in approximately ¥10,000 (nearly $1390) per patient annually (data not shown). Additionally, reductions of CsA dose and the incidence of hepatotoxicity and nephrotoxicity episodes lead to a decreased cost for prevention and treatment of toxicity, further resulting in a reduction of the total treatment cost for kidney allograft recipients.
Our results indicate that concurrent use of diltiazem and CsA not only reduces the oral CsA dosage, but also decreases the occurrence of hepatotoxicity and nephrotoxicity episodes. Co-administration of diltiazem and cyclosporine in kidney allograft recipients allows up to a 10–40% reduction of CsA dose although CsA serum levels may be reduced in over 1-year follow-up. This combination of CsA and diltiazem also results in a significant reduction of nephrotoxicity episodes. Concurrent use of diltiazem and CsA can significantly increase the patient and allograft survival compared to CsA use alone. These findings suggest that diltiazem is a safe, reliable, and excellent calcium antagonist that allows less CsA to be used in the allograft recipients and being beneficial to the clinical outcome of kidney transplantation without reported side effects.
Acknowledgments
This study was supported by National Natural Science Foundations of China in 2002 (30271241) and 2004 (30471640). We thank Dr. Pengbo Liu at the Clinical Epidemiological Center of the First Hospital at Xi'an Jiaotong University for helping with statistical analysis and the critical revision for this manuscript.
Declaration of interest: The authors report no conflicts of interest. We are alone responsible for the content and writing of this manuscript.
REFERENCES
- Hariharan S, Johnson CP, Bresnahan BA, Taranto SE, McIntosh MJ, Stablein D. Improved graft survival after renal transplantation in the United States, 1988 to 1996. N Engl J Med. 2000;342(9):605.
- Weir MR. Therapeutic benefits of calcium channel blockers in cyclosporine-treated organ transplant recipients: blood pressure control and immunosuppression. Am J Med. 1991;90(5A):32S.
- Kahan BD. Cyclosporine. N Engl J Med. 1989;321(25):1725.
- Chrysostomou A, Walker RG, Russ GR, d'Apice AJ, Kincaid-Smith P, Mathew TH. Diltiazem in renal allograft recipients receiving cyclosporine. Transplantation. 1993;55(2):300.
- Patton PR, Brunson ME, Pfaff WW, A preliminary report of diltiazem and ketoconazole. Their cyclosporine-sparing effect and impact on transplant outcome. Transplantation. 1994;57(6):889.
- Ingsathit A, Sumethkul V, Chalermsanyakorn P, Jirasiritham S. Co-administration of diltiazem and cyclosporine for kidney transplant recipients: A four year follow-up study. J Med Assoc Thai. 2006;89(Suppl. 2):S235.
- Wagner K, Albrecht S, Neumayer HH. Prevention of posttransplant acute tubular necrosis by the calcium antagonist diltiazem: A prospective randomized study. Am J Nephrol. 1987;7(4):287.
- Martin JE, Daoud AJ, Schroeder TJ, First MR. The clinical and economic potential of cyclosporin drug interactions. Pharmacoeconomics. 1999;15(4):317.
- Jones TE. Survey of cyclosporin-sparing agent use in Australasian transplant centres. Aust N Z J Med. 1996;26(6):772.
- Racusen LC, Solez K, Colvin RB, The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999;55(2):713.
- Grino JM, Sabate I, Castelao AM, Alsina J. Influence of diltiazem on cyclosporin clearance. Lancet. 1986;1(8494):1387.
- Bleck JS, Thiesemann C, Kliem V, Diltiazem increases blood concentrations of cyclized cyclosporine metabolites resulting in different cyclosporine metabolite patterns in stable male and female renal allograft recipients. Br J Clin Pharmacol. 1996;41(6):551.
- Halloran P, Mathew T, Tomlanovich S. Mycophenolate mofetil in renal allograft recipients: a pooled efficacy analysis of three randomized, double-blind, clinical studies in prevention of rejection. The International Mycophenolate Mofetil Renal Transplant Study Groups. Transplantation. 1997;63(1):39.
- Sollinger HW. Mycophenolate mofetil for the prevention of acute rejection in primary cadaveric renal allograft recipients. U.S. Renal Transplant Mycophenolate Mofetil Study Group. Transplantation. 1995;60(3):225.
- Pascual M, Theruvath T, Kawai T. Strategies to improve long-term outcomes after renal transplantation. N Engl J Med. 2002;346(8):580.
- Nankivell BJ, Borrows RJ, Fung CL, O'Connell PJ, Chapman JR, Allen RDM. Calnineurin inhibitor nephrotoxicity: longitudinal assessment by protocol histology. Transplantation. 2004;78(4):557.
- Li Y, Xue WJ, Tian PX, Clinical application of Cordyceps sinensis on immunosuppressive therapy in renal transplantation. Transplant Proc. 2009;41(5):1565.
- McCauley J, Ptachcinski RJ, Shapiro R. The cyclosporine-sparing effects of diltiazem in renal transplantation. Transplant Proc. 1989;21(6):3955.
