Abstract
Aim: Quantity of oxidative stress (OS) is enhanced in every stage of chronic renal failure (CRF). OS and its effects on echocardiographic indexes in patients on hemodialysis (HD) and continuous ambulatory peritoneal dialysis (CAPD) were evaluated. Materials and methods: Thirty-nine patients on CAPD, 32 patients on HD, and 30 healthy individuals with similar demographic features were included. Patients with diabetes mellitus and chronic inflammatory diseases were excluded. Blood samples were collected to examine hematological and biochemical parameters and levels of malonyldialdehyde (MDA), glutathione peroxidase (GSH-px), and superoxide dismutase (SOD) after a 12-hour fasting period in the middle of dialysis week. OS parameters were compared with ejection fraction (EF), interventricular septum diameter (IVSd), left ventricular posterior wall diameter (LVPWd), and left atrium diameter (LAd) determined in M-mod echocardiographic examination. Results: No significant difference was observed between MDA and GSH-px levels of patients and control group; however, SOD levels of patients group were significantly lower (p < 0.0001). SOD levels of patients on HD were lower than that of patients on CAPD (p = 0.039). Negative correlation was detected between MDA and EF (r = −0.380, p = 0.001); SOD has negative correlation with systolic blood pressure (r = −0.265, p = 0.011), diastolic blood pressure (r = −0.230, p = 0.028), phosphorus (r = −0.327, p = 0.001), intact parathyroid hormone (iPTH) (r = −0.259, p = 0.013), C-reactive protein (CRP) (r = −0.235, p = 0.024), fibrinogen (r = −0.342, p = 0.001), and total cholesterol (r = −0.249, p = 0.017); and positive correlation with hemoglobin (r = 0.414, p < 0.001) and albumin (r = 0.367, p < 0.001). MDA was independently related with age (β = −0.258, p = 0.035), male gender (β = −0.312, p = 0.004), and EF (β = −0.461, p < 0.001). No correlation was determined between antioxidants and cardiac indexes. Conclusion: SOD levels decreased significantly especially in patients on HD, and it was observed that lower levels of SOD would lead to OS in patients on HD and CAPD when compared to healthy individuals; MDA levels were independently influenced from EF.
INTRODUCTION
Atherosclerosis and associated cardiovascular diseases (CVD) are the most common causes of mortality and morbidity in end-stage renal disease (ESRD).Citation1 Risk of CVD is 3.5–50 times higher in patients undergoing renal replacement therapy than in general population.Citation2,Citation3 Augmentation in the risk of CVD in patients with ESRD is not only related to traditional risk factors, even when the traditional risk factors are fixed, but also related to untraditional and uremia-specific risk factors such as hypertension secondary to chronic hypervolemia, anemia, disorders of calcium–phosphorus (Ca–P) metabolism, hypercatabolism, hyperhomocysteinemia, and chronic microinflammation subsequent to oxidative stress (OS).Citation4
OS is described as dominance of pro-oxidants to antioxidant systems and plays an important role in the development of systemic diseases and related complications.Citation5,Citation6 It was concluded that proinflammatory cytokines and OS are cardinal factors for atherosclerosis in uremic patients. In patients with ESRD, OS is enhanced because of increased lipid peroxidation and decreased antioxidant activity that reflects basic properties of oxidative damage.Citation7
Several studies mentioning the relation of ESRD and OS have been carried out; however, few studies examined the relation between OS and dialysis modalities and its effects on echocardiographic indexes of left ventricular hypertrophy.
In this study we aimed to evaluate the relation between OS and dialysis modalities and its effects on echocardiographic parameters such as ejection fraction (EF), interventricular septum diameter (IVSd), left ventricular posterior wall diameter (LVPWd), and left atrium diameter (LAd) in patients on hemodialysis (HD) and continuous ambulatory peritoneal dialysis (CAPD).
MATERIALS AND METHODS
Thirty-nine patients on CAPD and 32 patients on HD followed up for at least 6 months period in the Dialysis Center of Medical School, Dicle University, and age-matched 30 healthy individuals were included into the study. The study was planned as a cross-sectional study and carried out in two stages. In the first stage, the patients and control groups were constituted. Parameters of biochemistry and OS were examined in both groups. In the second stage, the patients were divided into subgroups according to dialysis modality: HD (Group 1) and peritoneal dialysis (Group 2). Blood pressure of patients was measured after a period of 15 min resting and before dialysis in HD patients and after 15 min resting among CAPD patients. Clinical, hematological, biochemical examination, and the parameters of OS were evaluated and the effects of OS on echocardiographic indexes were determined. Patients with diabetes mellitus, chronic infection, or inflammation status (such as tuberculosis, rheumatoid arthritis, and amyloidosis), ischemic heart disease, congestive heart failure, who were hypertensive and were on antihypertensive therapy, and who refused to participate in the study were excluded. Written informed consent had been taken from all patients.
Collection of blood samples
Blood samples were collected from antecubital vein subsequent to a 12-hour fasting period before dialysis therapy in patients on HD and first removal in the morning among patients on CAPD. A pair of sterile biochemistry tubes including no additives, for parameters of OS and biochemical analysis and a hemogram tube including K-EDTA for erythrocyte superoxide dismutase (SOD) and glutathione peroxidase (GSH-px) examination, were prepared. Samples were centrifuged in 3000 turn for 15 min in room temperature. Serum samples and erythrocyte packages were collected in Eppendorf tubes and stored in −80°C to examine parameters of OS. Malonyldialdehyde (MDA), SOD, and GSH-Px analyses were studied in Biochemical Laboratory of Medical School in Harran University. Routine biochemical analysis was carried out in Central Laboratory of Medical School in Dicle University.
Malonyldialdehyde
Samples were stored in −80°C to examine plasma levels of MDA. Briefly, 50 μL of plasma was added to 1 mL of 10 mmol/L diethylthiobarbituric acid (DETBA) reagent in phosphate buffer (0.1 mol/L, pH 3). The mixture was mixed for 5 s and incubated for 60 min at 95°C. Samples were placed in ice for 5 min and then added to 5 mL of butanol. The mixture was shaken for 1 min to extract the DETBA–MDA adduct and then centrifuged at 1500 × g for 10 min at 4°C. Fluorescence of the butanol extract was measured at excitation wavelength of 539 and emission wavelength of 553. 1,1,3,3-Tetraethoxypropane (Sigma-Aldrich®, St. Louis, MO, USA) was used as a standard solution, and the values were presented as μmol/L.Citation8,Citation9
Erythrocyte superoxide dismutase activity
Erythrocyte levels of SOD were measured spectrophotometrically using Randox Lab. Ransod® (No. SD 125 Randox Laboratories, Diamond Road, Crumlin, UK) trademark kit.Citation10
Glutathione peroxidase
Levels of GSH-px were measured by modified method of Paglia Valentine enzymatic-UV using Ransel kit (catalog no: RS 504; Randox Laboratories) spectrophotometrically.Citation11
Echocardiographic analysis
Echocardiographic indexes were assessed using Hewlett–Packard Sonos 4500® Echocardiography (Philips, Andover, MA, USA) system transthoracically. Echocardiographic examination was instituted in two-dimensional M-mod, C-Doppler echocardiography in left lateral decubitus position, and in the assistance of parasternal long–short axis, apically four-space and five-space view. According to the recommendations of American Echocardiography Association, entire echocardiographic examination was done by the same physician in the middle of the day to prevent circadian changes. Measurements of EF, IVSd, LVPWd, and LAd were instituted in M-mod echocardiographic examination subsequent to dialysis period.
Statistical analysis
Statistical analysis was carried out using SPSS 13.0 PC program. Independent variables and dependent variables were determined by Student's t-test and linear regression methods, respectively, and the relation of variables was determined by Pearson's correlation. A p-value < 0.05 was accepted as significant, and data were shown as ±SD.
RESULTS
Patients on HD (n = 32), CAPD (n = 39), and healthy controls (n = 30) – totally 101 individuals – were included in the study. Demographic, clinical, and biochemical properties of patients and control group were compared and shown in .
TABLE 1. Demographic clinical and biochemical properties of patients and control group
No significant difference was observed between patients and control group in age, sex, levels of glucose, Na, Ca, total cholesterol, triglyceride, and low-density lipoprotein (LDL)-c; however, there was significant difference between other parameters.
Patients were divided into two subgroups as HD and CAPD, and demographic, clinical features and laboratory examination were compared as shown in .
Between subgroups (HD and CAPD patients), no significant difference was observed in age, sex, BMI, blood pressure, Na, hemoglobin, ferritin, Ca, parathyroid hormone (PTH), C-reactive protein (CRP), and triglyceride levels. Kt/V values were 1.46 ± 0.15 among HD patients and 2.13 ± 0.09 among CAPD patients. Although serum levels of phosphorus (p = 0.002), Ca × P (p = 0.036), albumin (p = 0.009), and high-density lipoprotein (HDL)-c (p < 0.001) were significantly higher in patients on HD, serum levels of glucose (p = 0.010), potassium (p = 0.002), total cholesterol (p = 0.012), and LDL-c (p = 0.020) were significantly higher in patients on CAPD.
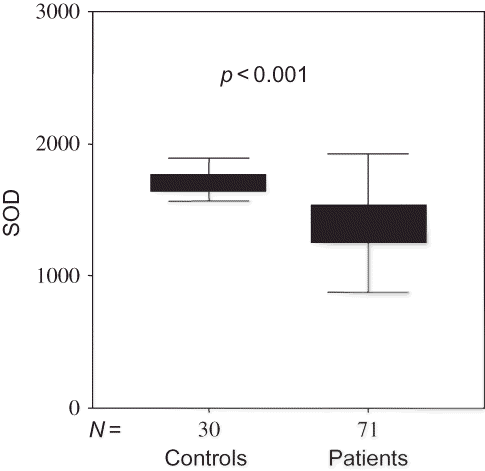
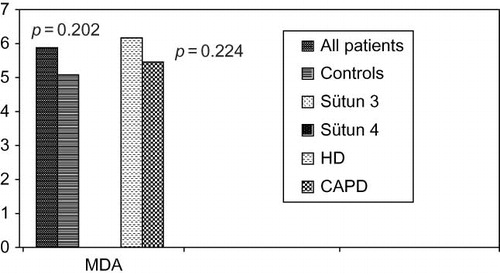
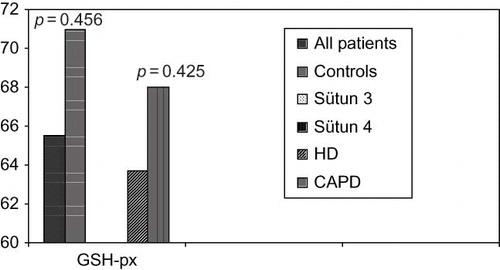
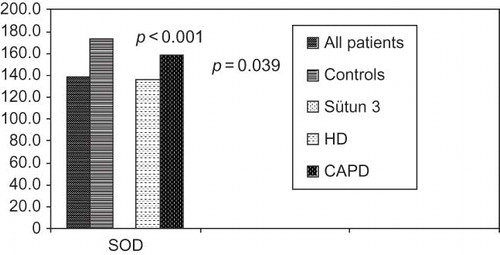
Although there was no significant difference between patients and control groups in the levels of MDA as pro-oxidant and GSH-px as antioxidant (p = 0.202 vs. p = 0.456), erythrocyte SOD levels were significantly different between groups (p < 0.001) (). OS parameters between patients and controls were compared in .
TABLE 2. Parameters of pro-oxidant and antioxidants between patients and controls
Among subgroups of patients according to dialysis modality, there was no significant difference between MDA (p = 0.224) and GSH-px (p = 0.425) levels. Significant difference was determined in SOD levels (p = 0.039). According to the OS effects of pro-oxidant (MDA) and antioxidant (GSH-px), results were relatively better in patients on CAPD than patients on HD, even it was insignificant as shown in and .
TABLE 3. Oxidative stress and echocardiographic data
FIGURE 4. Comparison of superoxide dismutase levels between patients on HD, SAPD, and control groups.
No statistical significance was found between GSH-px and SOD levels when OS parameters and echocardiographic indexes were taken into account. There was a negative correlation between MDA levels and EF (r = −0.380, p = 0.001). Also negative correlations were determined between SOD and systolic blood pressure (r = −0.265, p = 0.011), diastolic blood pressure (r = −0.230, p = 0.028), biochemical parameters such as p (r = −0.327, p = 0.001), PTH (r = −0.259, p = 0.013), CRP (r = −0.235, p = 0.024), fibrinogen (r = −0.342, p = 0.001), total cholesterol (r = −0.249, p = 0.017). Results were detailed in . Positive correlation was determined between SOD and hemoglobin (r = 0.414, p < 0.001) or albumin (r = 0.367, p < 0.001).
TABLE 4. Results of Pearson's correlation
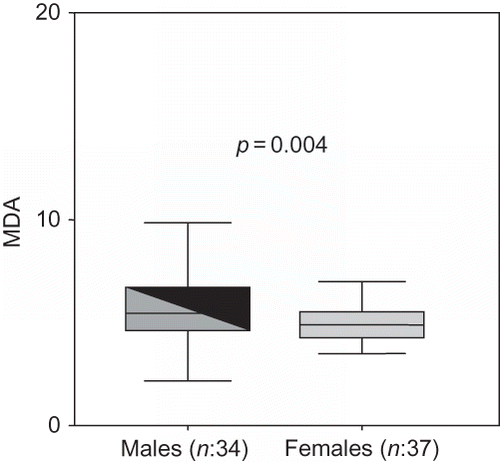
MDA was independently correlated with age (β = −0.258, p = 0.035) and male gender (β = −0.312, p = 0.004) (). MDA was the only antioxidant or pro-oxidant parameters that has significant correlation with echocardiographic indexes, especially with EF (β = −0.461, p < 0.001). No correlation was detected between antioxidants (GSH-px or SOD) and cardiac parameters.
Linear regression analysis pointed out positive correlation between MDA, as dependent variable, and echocardiographic indexes like EF, LAd, IVSd versus LVPWd (r = 0.189 and p = 0.007), as independent variables. Other OS parameters and echocardiographic indexes have no significant correlation.
DISCUSSION
ESRD is usually collaborated with premature atherosclerosis. This is generally committed to nontraditional risk factors such as hypervolemia, anemia, Ca–P metabolism disorders, hyperhomocysteinemia, and chronic inflammation besides traditional risk factors.Citation12 Recently, role of OS as a nontraditional risk factor is prominent. Association of OS and carotid artery intima-media thickness accepted as earlier predictor of atherosclerosis,Citation12,Citation13 related to OS and CRP that is an independent risk factor of atherosclerotic heart disease, and oxide-LDL cholesterol deposition in foam cells that forms atheromatous plaque are the factors suggesting the role of OS in atherosclerotic process.
OS is a process resulting in tissue damage subsequent to the elevation of pro-oxidants and the reduction of antioxidant or both that is physiologically balanced.Citation14 ESRD is a disorder associated with OS that is not totally enlightened. Possibly, OS has a multifactorial origin in ESRD. Quantity of OS is enhanced because of inadequate clearance of OS substances, use of bioincompatible membranes, dialysate reactions, reduced production of antioxidants, HD itself that is a model for OS, and iron replacement that would precipitate OS.
Elevated quantity of OS is attributed to lipid peroxidation, endothelial dysfunction, and increased modification of proteins. Elevated levels of MDA reflects elevated lipid peroxidationCitation15 as a marker of enhanced OS. In recent study there was no statistical significance between patients undergoing HD and CAPD (p = 0.224).
GSH-px, an enzyme which has antioxidant effect, is secreted from proximal tubule cells and erythrocytes, plays a critical role in antioxidant defense system of plasma.Citation16 Insignificant reduction levels of GSH-px were determined in patients on HD when compared with control group (p = 0.456) and in patients on CAPD when compared with HD (p = 0.425). Although contradictious results are present, most of other studies support our data.Citation17–20
Erythrocytes SOD levels, a parameter of antioxidant defense system, was significantly lower in patients on dialysis therapy than control group and in patients on HD than CAPD (p < 0.001 and p = 0.039, respectively). Statistically no significant difference was observed in levels of GSH-px between patients and control group and between patients on HD and CAPD. Similar results were observed in some other reports.Citation21 It is associated with adequacy of dialysis (Kt/V = 1.68), acceptable levels of anemia, secondary hyperparathyroidism, and parameters of nutrition such as albumin and cholesterol. Many studies reflecting similar results are present.Citation22–26
Antioxidant defense systems are less affected in patients on peritoneal dialysis than HD.Citation15–Citation17 This is related to less frequent presence of anemia and moderate amount of lipid peroxidation.Citation23 Similarly, levels of SOD enzyme were significantly lower in patients on CAPD than HD. Parameters of antioxidant defense system were better in patients on CAPD according to extracorporeal circulation and elevated activation of immune system in patients on HD. This is attributed to the adequacy of dialysis and better results of other related parameters in patients on CAPD as it was same as HD. OS is a higher potential risk factor for patients on HD; however, it was also elevated in patients on CAPD. Elevation occurs independently from therapy in uremic patients as it was supported by many other reports.Citation12,Citation16,Citation27
Negative correlation between SOD and biochemical parameters like P, PTH, CRP, fibrinogen, and total cholesterol and positive correlation between hemoglobin (r = 0.414, p < 0.001) and albumin (r = 0.367, p > 0.0019) were determined. Relation between OS parameters and both CRP, as an inflammation marker, or secondary hyperparathyroidism and nutritional factors, indicate not only elevation of pro-oxidants but also synchronous insufficiency of antioxidant defense systems. Several studies supporting this data are observed in the literature.Citation28
Chronic renal failure (CRF) is common due to frequent association of hypertension and etiological factors including diabetes mellitus, chronic glomerulonephritis, polycystic kidney disease, or left ventricular hypertrophy both secondary to hypertension or subsequent to anemia, secondary hyperparathyroidism, and activation of renin–angiotensin–aldosterone system.Citation29 Several studies pointed out the association of LVH and stroke, acute coronary syndromes, heart failure, and sudden death. LVH is an independent risk factor for increased incidence of CVD besides coronary artery disease, heart failure, stroke, and mortality secondary to both cardiac and noncardiac causes in male and female patients. LVH is described according to the relation of mass of left ventricle and relative wall thickness. Echocardiography is the most common diagnostic procedure. Increase of left ventricular mass in hypertensive patients has predictive value and better predictor of cardiac complication.Citation30,Citation31 OS is related with cardiovascular damage in earlier and late stages of CRF in both adults and pediatric population.Citation27,Citation32 In our study, no statistical significance was observed between echocardiographic indexes and plasma GSH-px; however, negative correlation between MDA and EF (r = −0.380, p < 0.001) or between SOD and systolic-diastolic blood pressure suggests particular role of OS on left ventricle hypertrophy. Positive relation of MDA, as an independent variable, and echocardiographic indexes like EF, LAd, IVSd, and LVPWd, as a dependent variable (r = 0.189 and p = 0.007) supports our data in linear regression analysis. We suggest that favorable results are related to adequate dialysis therapy for more than 6 months and better control of anemia, hypervolemia, inflammation, nutritional status, Ca–P metabolism, dyslipidemia, and hypertension.
In conclusion, SOD levels diminished and caused OS in ESRD patients on CAPD and particularly HD according to control group. MDA levels independently correlates with age, male gender, and EF, and finally SOD levels have negative correlation with blood pressure, P, PTH, CRP, fibrinogen, and positive correlation with hemoglobin and albumin.
Acknowledgments
Authors thank Sakir Altintas for his grateful support on language. Also, the authors thank Prof. Dr. Ozcan Erel and Dr. Sahbettin Selek from the Department of Biochemistry in Medical School, Harran University, and Assoc. Prof. Dr. Sait Alan from the Department of Cardiology in Medical School, Dicle University, for their grateful contribution to this study.
Declaration of interest: The authors report no conflicts of interest, and declared that no funding has been received from anywhere.
REFERENCES
- Locatelli F, Marcelli D, Conte F, Cardiovascular disease in chronic renal failure: The challenge continues. Nephrol Dial Transplant. 2000;15(Suppl. 5):S69–S80.
- Levey AS, Beto JA, Coronado BE, Controling the epidemic of cardiovascular disease in chronic renal disease: What do we know? What do we need to learn? Where do we go from here? Am J Kidney Dis. 1998;32:853–906.
- Sit D, Kadiroglu AK, Kayabasi H, The evaluation incidence and risk factors of mortality among patients with end stage renal disease in Southeast Turkey. Ren Fail. 2008; 30(1):37–44.
- Zoccali C. Cardiovascular risk in uraemic patients – is it fully explained by classical risk factors? Nephrol Dial Transplant. 2000;15:454–457.
- Ronco C, La Greca G. Vitamin E bonded membrane, a further step in dialysis optizimation. Contrib Nephrol. 1999;127:1–31.
- Sies H. Oxidants and antioxidants. Exp Physiol. 1997;82:291–295.
- Boaz M, Matas Z, Biro A, Serum malondialdehyde and prevalent cardiovascular disease in hemodialysis. Kidney Int. 1999;56:1078–1083.
- Harma M, Harma M, Kocyigit A, Erel O. Increased DNA damage in patients with complete hydatiform mole. J Mutat Res. 2005;583:49–54.
- Conti M, Morand PC, Levillain P, Lemonnier A. Improved fluorimetric determination of malondialdehyde. Clin Chem. 1991;37(7):1273–1275.
- McCord JM, Fridovich I. Superoxide dismutase. An enzymatic function for erytrocuprein (hemocuprein). J Biol Chem. 1969;244:6049–6055.
- Paglia DE, Valentina WN. Glutathione peroxi-dase. J Lab Clin Med. 1967;70:158.
- Levin A. Clinical epidemiology of cardiovascular disease in chronic kidney disease prior to dialysis. Semin Dial. 2003;16(2):101–105.
- Drueke T, Witko-Sarsat V, Massy Z, Iron therapy, advanced oxidation protein products, and carotid artery intima-media thickness in end-stage renal disease. Circulation. 2002;106:2212–2217.
- Tsirpanlis G. Cellular senescence, cardiovascular risk, and CKD: A review of established and hypothetical interconnections. Am J Kidney Dis. 2008;51(1):131–144.
- Matteucci E, Cupisti A, Caprioli R, Erythrocyte transmembrane electron transfer in hemodialysis patients. Nutr Metab Cardiovasc Dis. 2007;17(4):288–293.
- Avissar N, Ornt BA, Yagil Y, Horowitz S. Human kidney proximal tubules are the main source of plasma glutathione peroxidase. Am J Physiol. 1994;266:367–375.
- Çeliker H, Elkıran B, İlhan N, Günal Aİ, Doğukan A. The effects of hemodialysis and peritoneal dialysis on oxidative stres parameters. Official J Turk Soc Nephrol. 2001;10(2):88–92.
- Köse K, Doğan P, Gündüz S, Düşünsel R, Utaş C. Oxidative stress in hemodialysis patients and long term effects of dialyzer reuse practice. Clin Biochem. 1997;30:601–606.
- Wu CC, Chen JS, Wu WM, Myeloperoxidase serves as a marker of oxidative stress during single hemodialysis session using two different biocompatible dialysis membranes. Nephrol Dial Transplant. 2005;20(6):1134–1139.
- Ilgen C, Bulucu F, Yamanel L, The effect of different dialyzer membranes on the oxidative burst status of polymorphonuclear leukocytes in hemodialysis patients. Official J Turk Soc Nephrol. 2007;16(2):63–67.
- Johnson DW, Armstrong K, Campbell SB, Metabolic syndrome in severe chronic kidney disease: Prevalence, predictors, prognostic significance and effects of risk factor modification. Nephrology (Carlton). 2007;12(4):391–398.
- Cristol JP, Bose JY, Badiou S, Erythropoietin and oxidative stress in hemodialysis. Benefical effects of vitamin E supplementation. Nephrol Dial Transplant. 1997;12:2312–2317.
- Rysz J, Stolarek RA, Pedzik A, Increased exhaled H2O2 and impaired lung function in patients undergoing bioincompatible hemodialysis. Int J Artif Organs. 2007;30(10):879–888.
- Biasioli S, Schiavon R, Petrosino L, Free radicals and oxidative stress challenge dialysis patients: Effects of two different membranes. ASAIO J. 1997;43(5):M766–M772.
- Al-Hashimi AF, Mohammed FH, Al-Khazragi AS. Oxidative stress in chronic renal failure patients treated by peritoneal dialysis. Saudi Med J. 2004;25(9):1186–1192.
- Yano S. Parathyroid and bone. Mineral and bone disorder and parathyroid function in chronic kidney disease. Clin Calcium. 2007;17(12):1870–1878.
- Pawlak K, Pawlak D, Mysliwiec M. Method of dialysis therapy and selected markers of oxidative stress and endothelial injury in patients with chronic renal failure. Pol Arch Med Wewn. 2005;113(1):21–26.
- Yilmaz MI, Carrero JJ, Axelsson J, Lindholm B, Stenvinkel P. Low-grade inflammation in chronic kidney disease patients before the start of renal replacement therapy: Sources and consequences. Clin Nephrol. 2007;68(1):1–9.
- Scott B, Deman A, Peeters P, Cardiac troponin T and malondialdehyde modified plasma lipids in hemodialysis patients. Nephrol Dial Transplant. 2003;18(4):737–742.
- Ece A, Gürkan F, Kervancioğlu M, Oxidative stress, inflammation and early cardiovascular damage in children with chronic renal failure. Pediatr Nephrol. 2006;21(4): 545–552.
- Gugliucci A, Mehlhaff K, Kinugasa E, Paraoxonase-1 concentrations in end-stage renal disease patients increase after hemodialysis: Correlation with low molecular AGE adduct clearance. Clin Chim Acta. 2007;377(1–2):213–220.
- Liao Y Cooper RS, McGee DL, Mensah G, Ghali JK. The relative effects of left ventricular hypertrophy, coronary artery disease, and ventricular dysfunction on survival among black adults. JAMA. 1995;273:1592–1597.