Abstract
Peritonitis accounts for considerable morbidity and hospitalization in peritoneal dialysis (PD) patients. We investigated the factors related with time of hospital stay, especially focusing on the peritoneal cell profiles at the time of hospital admission in PD-related peritonitis. Eighty peritonitis attacks were evaluated. Data were collected at the time of hospital admission, clinical and biochemical parameters, including initial systemic and dialysate white cell counts (including percentage and differential count of neutrophils and lymphocytes) and length of hospital stay. Peritoneal leukocyte (r = +0.289, p = 0.009) and neutrophil counts (r = +0.403, p < 0.0001), peritoneal neutrophil percentage (r = +0.492, p < 0.0001), time of hospital admission (r = +0.498, p < 0.0001), and C-reactive protein (CRP) (r = +0.231, p = 0.042) were positively correlated; lymphomononuclear cell percentage (r = −0.650, p < 0.0001) was negatively correlated with hospitalization length. Hospital admission ≥24 hours of symptom onset was associated with higher CRP, dialysate leukocyte and neutrophil counts, longer hospitalization, and with lower dialysate lymphomononuclear cell percentage compared to admission < 24 hours (p = 0.04, p = 0.04, p = 0.005, p < 0.0001, and p = 0.04, respectively). In multiple linear regression, the time of hospital admission (p = 0.002), initial peritoneal neutrophil count (p = 0.011), and lymphomononuclear cell percentage (p < 0.0001) were independently associated with hospitalization length. Hospital admission within first 24 hours of peritonitis symptoms onset is of vital importance; delayed admission is associated with higher peritoneal leukocyte and neutrophil counts, and increased length of hospital stay.
INTRODUCTION
The technique for peritoneal dialysis (PD) was first described by Popovich et al. in 1976. Since then, its use has increased rapidly, and, worldwide, the annual estimated growth of continuous ambulatory peritoneal dialysis (CAPD) is 12%.Citation1 The most common complication of PD therapy is peritonitis. Although there have been improvements in CAPD techniques (including the Y-set and twin-bag systems) and patient education, recurrent peritonitis still remains the most common complication of CAPD therapy.Citation1,Citation2–4 Peritonitis is an important clinical problem in automated peritoneal dialysis (APD) patients as well.Citation5
The inflammatory response occurring during infection (peritonitis) is a multifaceted sequence of controlled events. During episodes of acute bacterial peritonitis, a characteristic profile of leukocyte trafficking is normally observed, with massive elevation in the number of neutrophils in the first 24 hours of infection, which then decreases over time; the subsequent “switch” to lymphomononuclear cell influx is in evidence between days 4 and 5 of infection.Citation6 Thus, there is a bimodal recruitment pattern during acute peritoneal infection/inflammation involving a switch from neutrophils to lymphomononuclear cells.Citation7 Any interruption or breakdown in this precise and highly regulated network may result in a loss of control of the peritoneal innate immune response, which might potentially result in retention of the acute inflammatory infiltrate and/or in the maintenance of more chronic inflammatory exudates (mononuclear cell and lymphocyte) within the peritoneal cavity.Citation6
The outcome of peritonitis can potentially be influenced by several factors, including microbial etiology, host factors, and interventional factors.Citation8 Microbial etiology and antibiotic regimens have been extensively studied in the literature.Citation8,Citation9 Unfortunately, host factors have received inadequate attention.Citation8 The primary aim of this study was to investigate the factors related to the time of hospital stay, especially focusing on the peritoneal cell profiles at the time of hospital admission in patients with PD-related peritonitis.
MATERIALS AND METHODS
End-stage renal disease patients who were on PD therapy in Baskent University Hospital's Peritoneal Dialysis Unit and who suffered from peritonitis were included. The research was conducted in accordance with the principles set forth in the Helsinki Declaration (http://www.wma.net/e/policy/b3.htm) and was approved by the institutional ethical committee.
All patients on CAPD therapy used a double-bag system with a titanium connector (Baxter, Dianeal 137) and all patients on APD used a HomeChoice cycler device (Baxter Healthcare Corporation, Deerfield, Illinois, USA). In our center, the initial and follow-up protocol of PD therapy includes the patients' and the family members' standard training in the first 3 weeks of the therapy by our staff.
PD patients, either on CAPD or on APD therapy, who had suffered from peritonitis, were included in the study. Exclusion criteria were peritonitis caused by fungi (because of a general high nonresolution rate and an indication for catheter removal) or Mycobacterium tuberculosis, presence of catheter exit site and/or tunnel infection (ruled out by radiological evaluation), steroid use (for any indication) during the peritonitis attack, history of renal transplantation, and presence of malignancy.
The time elapsed between the onset of symptoms of peritonitis and hospital admission was determined for each patient. The data including age, gender, weight (with an empty abdomen), height, PD vintage, PD modality, and the etiology for end-stage renal disease were noted. Body mass index was calculated according to the following formula:
Peritonitis was diagnosed according to the criteria defined by the Ad Hoc Advisory Committee on Peritonitis Management. Two of the three following criteria had to be fulfilled: (i) 100 or more white blood cells (WBC)/mm3 of dialysate; (ii) clinical manifestations of peritonitis (a cloudy dialysate or abdominal pain or fever); and (iii) positive dialysate culture.Citation10 Episodes of recurrent/relapsing, repeat, or polymicrobial peritonitis were determined.Citation11
In all patients who had been admitted with signs and symptoms of peritonitis, the abdomen was drained and the effluent was carefully inspected and sent for cell count (with differential), Gram stain, and culture. Patients on APD with a day dwell who presented during the day had cell counts similar to those on CAPD. In APD patients without a day dwell who presented with abdominal pain, 1 L of dialysate was infused and permitted to dwell a minimum of 1–2 hours, and then drained and examined for turbidity and sent for cell count (with differential), Gram stain, and culture. In equivocal cases, or in patients with systemic or abdominal symptoms in whom the effluent appears clear, a second exchange is performed with a dwell time of at least 2 hours.Citation12 For bacterial culture of PD effluent, freshly drained effluent was analyzed. Isolation and identification were performed using standard techniques.
For each episode of peritonitis, data were collected on the condition of the exit site, blood urea nitrogen, serum creatinine, total protein, albumin, electrolyte concentrations, C-reactive protein (CRP), hemoglobin, hematocrit, systemic WBC count (including percentage and differential count of neutrophils and lymphocytes), initial PD effluent white cell count (including percentage and differential count of neutrophils and lymphocytes), causative microorganism, presence of recurrent/relapsing or repeat peritonitis, and length of hospital stay due to peritonitis.
Following the diagnosis of peritonitis, all patients were hospitalized and were switched to standard (manual) CAPD procedure with a 4–6 hours dwell time. According to our protocol, all patients with peritonitis have been treated initially with empirical ampicillin–sulbactam and ciprofloxacin; thereafter antibiotic therapy was tailored to the results of bacteriological sensitivities.
Serum albumin level was measured by means of the bromocresol green method. A colorimetric method was used to measure serum calcium and phosphorus (Beckmann C × -7 autoanalyzer; Beckman Instruments Inc., Diagnostic Systems Group, Brea, California, USA). The turbidimetric latex agglutination method (Biosystems S.A., Barcelona, Spain) was used to determine CRP level. Other biochemical parameters were measured by means of standard laboratory methods.
Statistical analysis
Statistical analysis was performed using SPSS 11.5 for Windows (SPSS Inc., Evanston, Illinois, USA). The normality of the data was evaluated by the Kolmogorov–Smirnov test (Lilliefors modification). Data are shown as mean ± SD (normally distributed continuous variables), as median-range (nonnormally distributed continuous variables), and as a percentage (%). Pearson's and Spearman's correlation coefficients r were used for the analysis of continuous variables. Comparisons of the two groups were assessed by means of the T-test for normally distributed continuous variables and by the Mann–Whitney U-test for nonnormally distributed continuous variables. Multiple linear regression analysis was performed to detect the potential predictors of hospitalization duration following a peritonitis attack. Results were considered statistically significant if the two-tailed p-value was <0.05.
RESULTS
Totally, 80 peritonitis attacks of 55 patients included in the study (mean age, 44.5 ± 16.1 years; male/female ratio = 23/32) were investigated. Mean PD vintage and mean body mass index were 48.3 ± 23.8 months and 24.4 ± 4.9 kg/m2, respectively. Among 55 patients, 8 (14.5%) were on APD and 47 (85.5%) were on CAPD. Patients on APD were younger than patients on CAPD (p = 0.003) (data not shown).
The etiology for end-stage renal failure was unknown in 17 (30.9%) patients. Etiology was glomerulonephritis in 11 (20.0%), hypertension in 9 (16.4%), pyelonephritis in 5 (9.1%), polycystic kidney disease in 5 (9.1%), diabetes mellitus in 4 (7.3%), amyloidosis in 1 (1.8%), focal segmental glomerulosclerosis in 1 (1.8%), and miscellaneous in 2 (3.6%) patients.
During follow-up, 37 patients suffered from one attack, 13 patients suffered from two attacks, 4 patients suffered from three attacks, and 1 patient suffered from five attacks of peritonitis. The mean-time elapsed from the onset of clinical symptoms of peritonitis and hospital admission was 35.7 hours (median, 24 hours; minimum–maximum, 0–168 hours). summarizes the laboratory parameters in peripheral blood and in PD effluent at the time of hospital admission.
TABLE 1. Laboratory parameters of 80 peritonitis attacks of 55 patients at the time of hospital admission
The most common agents for peritonitis were Gram-positive microorganisms, which were isolated in 44 (55.0%) attacks. The most common causative Gram-positive agent was Staphylococcus epidermidis isolated in 28 (35.0%) attacks, followed by α-hemolytic streptococci isolated in eight (10.0%) attacks, and Staphylococcus aureus isolated in seven (8.7%) attacks. Peritoneal dialysate culture was negative in 23 (28.7%) attacks. Gram-negative agents were isolated in nine (11.3%) attacks, Escherichia coli in seven (8.7%) attacks, Pseudomonas aeruginosa in one (1.3%) attack, and Morganella morganii in one (1.3%) attack. The prevalence of polymicrobial peritonitis was 5.0% (4 attacks).
Recurrent/relapsing peritonitis was encountered in one patient and repeat peritonitis was encountered in five patients. In one patient with recurrent/relapsing peritonitis, the etiologic agent was S. aureus. In five patients with relapsing peritonitis, the etiologic agents were S. aureus in two patients, α-hemolytic streptococci in one patient, S. epidermidis in one patient, and E. coli in one patient.
The PD effluent leukocyte count at the time of hospital admission was positively correlated with the length of hospital stay (r = +0.289, p = 0.009) (). The neutrophil count and percentage in the PD effluent at the time of admission were positively correlated with the length of hospital stay (r = +0.403, p < 0.0001 and r = +0.492, p < 0.0001, respectively). The lymphomononuclear cell percentage in the dialysis effluent (r = −0.650, p < 0.0001) was negatively correlated with the length of hospital stay. demonstrates the correlations of neutrophil and lymphomononuclear cell percentages in the PD effluent at the time of hospital admission with clinical and laboratory parameters. The length of hospital stay was positively correlated with the time elapsed between the onset of symptoms of peritonitis and hospital admission (r = +0.498, p < 0.0001) and the serum CRP level at the time of admission (r = +0.231, p = 0.042). and demonstrate the regression graphics of these parameters.
FIGURE 1. The regression graphic of peritoneal dialysis effluent leukocyte count at the time of hospital admission and the length of hospital stay.
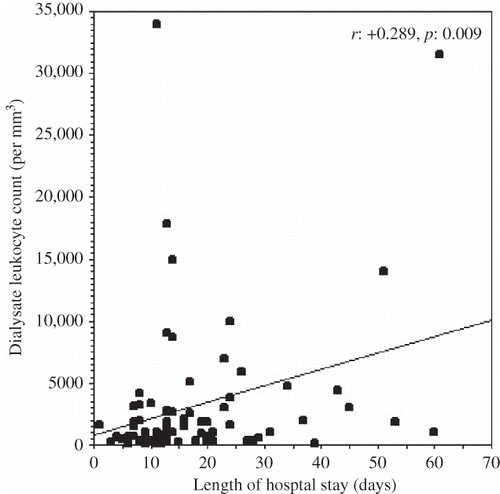
FIGURE 2. The regression graphic of the time elapsed between the onset of symptoms of peritonitis and hospital admission, and the length of hospital stay.
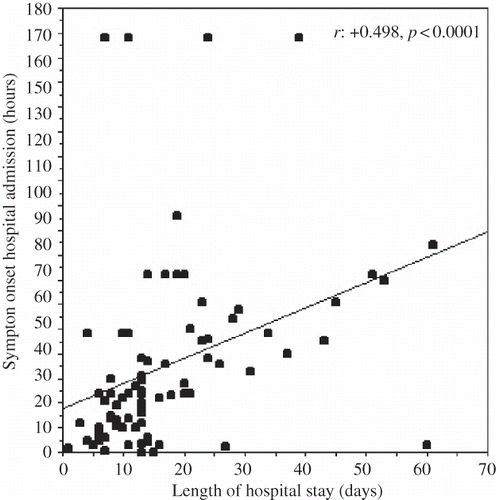
FIGURE 3. The regression graphic of serum C-reactive protein level at the time of hospital admission and the length of hospital stay.
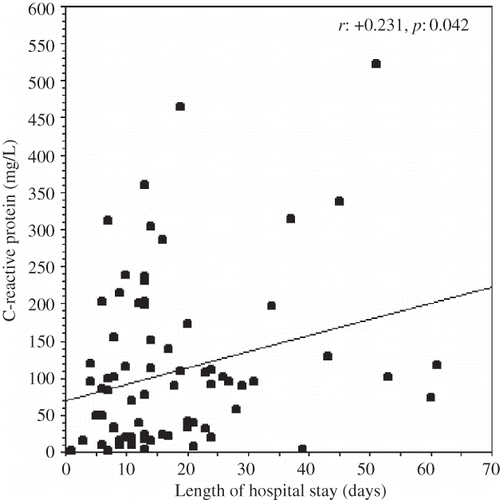
TABLE 2. The correlations of peritoneal dialysis effluent neutrophil and lymphomononuclear cell percentages at the time of hospital admission with clinical and laboratory parameters
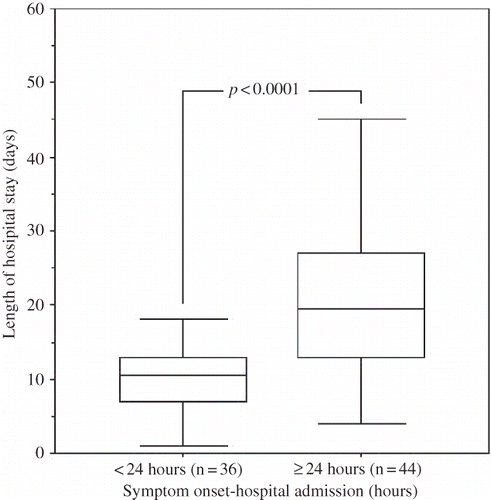
In only 36 of 80 attacks (45.0%), patients were admitted to the hospital within the first 24 hours of the onset of peritonitis symptoms. The time elapsed between the symptoms onset and the hospital admission was ≥ 24 hours in the remaining 44 attacks (55.0%). demonstrates the comparative clinical and laboratory data of the patients who had been admitted to the hospital within or ≥ 24 hours of onset of peritonitis symptoms.
TABLE 3. The comparative clinical and laboratory data of the patients who had been admitted to the hospital within or ≥24 hours of onset of peritonitis symptoms
In PD patients who had been admitted to the hospital ≥ 24 hours after the onset of symptoms of peritonitis, serum CRP levels, PD effluent leukocyte, and neutrophil counts were higher, and PD effluent lymphomononuclear cell percentage was lower than those patients who were admitted within the first 24 hours (p = 0.04, p = 0.04, p = 0.005, and p = 0.04, respectively) (). The length of hospital stay because of peritonitis was higher in patients who had been admitted ≥ 24 hours after the onset of symptoms of peritonitis than those who had been admitted within the first 24 hours ().
FIGURE 4. The comparisons of the lengths of hospital stay of patients who had been admitted to hospital within and ≥24 hours of the onset of peritonitis symptoms.
In the multiple linear regression analysis of factors present at the time of hospital admission (age, diabetes mellitus, dialysis vintage, time elapsed between the onset of peritonitis symptoms and hospital admission, albumin, CRP, PD effluent neutrophil count and lymphomononuclear cell percentage, and presence of polymicrobial peritonitis), time elapsed between onset of peritonitis symptoms and hospital admission (beta coefficient = 0.132, 95% confidence interval = 0.050–0.214, p = 0.002), PD effluent neutrophil count (beta coefficient = 0.001, 95% confidence interval = 0.000–0.001, p = 0.011), and PD effluent lymphomononuclear cell percentage (beta coefficient = −0.832, 95% confidence interval = −1.191–(−0.473), p < 0.0001) were independently related with the length of hospital stay because of peritonitis in PD patients ().
TABLE 4. Multiple linear regression analyses of potential predictors of the length of hospital stay because of a peritonitis attack
DISCUSSION
Although the use of CAPD therapy has increased rapidly in the recent years, peritonitis, which is the most common complication encountered during the therapy, still accounts for considerable morbidity and mortality.Citation1,Citation2–4 In this study, we investigated the association of time of hospital admission, initial (present at the time of hospital admission) peritoneal neutrophil and lymphomononuclear cell profiles, clinical and laboratory parameters with the course of peritonitis, and the length of hospital stay because of peritonitis in PD patients.
The typical spectrum of isolates during peritonitis include Gram-positive organisms (67%), Gram-negative organisms (28%), and fungal (2.5%) or anaerobic organisms (2.5%).Citation11 The most common pathogen found in PD-related peritonitis is S. epidermidis, which is responsible for 30–45% of peritoneal infections.Citation13 Staphylococcus aureus accounts for about 15% of the isolates.Citation11 In a study by Zelenitsky et al., 66.7% of peritonitis attacks were due to Gram-positive microorganism, whereas 28% of the attacks were due to Gram-negative microorganisms.Citation14 Korbet et al. and Bernardini et al. have reported much higher incidences of Gram-positive peritonitis (79 and 74.2%, respectively) and a lower incidence of Gram-negative peritonitis (16 and 12%, respectively).Citation15,Citation16 In our study of 80 peritonitis attacks, the most common pathogens were Gram-positive microorganisms (55.0%), and S. epidermidis (35%) was the most commonly isolated Gram-positive microorganism. Gram-negative organisms were isolated in 11.3% of the attacks. The spectrum of pathogens isolated in our study was similar to those previously reported in the literature.Citation11,Citation13 Krishnan et al. observed polymicrobial peritonitis in 10% of infections.Citation17 The incidence of polymicrobial peritonitis reported by Kim et al. and Bunke et al. was 16 and 14%, respectively.Citation18,Citation19 In our study, the incidence of polymicrobial peritonitis (5.0%) was relatively low compared to the reported incidence of 10% or higher in the literature.Citation17–19 The incidence of culture-negative peritonitis, which may happen for a variety of technical or clinical reasons, in series ranges from 13.7 to 21%.Citation4,Citation17 The incidence of culture-negative peritonitis is high in peritonitis attacks because of low virulence microorganisms or coagulase-negative staphylococci and may rise up to 20% in some series.Citation1,Citation11 In our study, the incidence of culture-negative peritonitis was 28.7%, which was relatively higher than reported in the literature.
In a study by Krishnan et al., the patients who had a successful outcome following bacterial peritonitis had been on CAPD for a significantly shorter period of time than those patients who had nonresolution. The nonresolution rate for those patients who had been on PD for more than 2.4 years was 24.4%, compared to 16.5% for those who had been on PD for less than 2.4 years.Citation17 In our study, the time on PD was not related with the length of hospital stay because of PD-related peritonitis. However, the time elapsed between the onset of symptoms and hospital admission (p = 0.002), PD effluent neutrophil count (p = 0.011), and lymphomononuclear cell percentage (p < 0.0001) at the time of hospital admission were independently related with the length of hospital stay.
The severity of the attack is an important predictor of morbidity following peritonitis and to prevent delay in treatment, antibiotic therapy should be initiated as soon as the cloudy effluent is seen, even without waiting for confirmation of the cell count from the laboratory.Citation6,Citation12 In our study, we demonstrated that more than one half (55.0%) of the PD patients were not able to admit to the hospital within the first 24 hours of symptom onset, which represents the time of transition from innate to acquired immunity, the pivotal element in the resolution of peritoneal inflammation. They were admitted to hospital in an average of 35.7 hours after the onset of symptoms and were able to receive treatment thereafter. As the time elapsed between the onset of symptoms and the hospital admission increased, the length of hospital stay increased accordingly (p < 0.0001) and the length of hospital stay was significantly higher in patients who had been admitted ≥ 24 hours of symptom onset than those who had been admitted within first 24 hours (p < 0.0001).
CRP is a marker of inflammation. Troidle et al. noted that the CRP values rose from a baseline value of 15.01 ± 11.05 to 118.35 ± 96.86 mg/dL 48 hours after the onset of peritonitis.Citation20 In most patients, CRP levels return to normal within the 4-week period after the onset of treatment for peritonitis. However, 20% of the patients might have persistently elevated CRP values 4 weeks after the onset of peritonitis, and 33% of these patients have repeated episodes of peritonitis.Citation21 In another study, Fontán et al. showed that the baseline risk of peritonitis-related mortality was significantly higher in patients with high baseline serum CRP levels.Citation22 In our study, serum CRP levels of PD patients who had been admitted to the hospital ≥24 hours of symptom onset were higher than those who had been admitted within the first 24 hours (p = 0.04), and as the CRP level at the time of hospital admission increased, the length of hospital stay increased accordingly (p = 0.042).
In a retrospective analysis of 627 PD patients by Cueto-Manzano et al., it was demonstrated that peripheral blood lymphopenia was a predictor of mortality. Although the role of lymphopenia was not clear, it was suggested that it might be related to a worse nutritional status or an altered immunological condition, which may decrease natural resistance to infection. They recommended that the total lymphocyte count be considered as an important predictive factor for CAPD outcome.Citation23 Additionally, it has been demonstrated that the longitudinal changes in peritoneal transport and ultrafiltration in CAPD patients correlated with cumulated WBC count during peritonitis, presumably as a result of unchecked mesothelial damage by neutrophils.Citation24 In PD-related peritonitis, the association of initial (present at the time of hospital admission) peritoneal neutrophil and lymphomononuclear cell profiles with the course of peritonitis and the length of hospital stay because of peritonitis is not exactly known. In our study, the initial PD effluent leukocyte count (p = 0.009), neutrophil count (p < 0.0001), and neutrophil percentage (p < 0.0001) were positively correlated with the length of hospital stay. The PD effluent lymphomononuclear cell percentage was negatively correlated with the length of hospital stay (p < 0.0001). Martikainen et al. studied 36 PD patients who had suffered from peritonitis and measured dialysate leukocyte count and serum CRP levels on days 1–4 of peritonitis. They showed that both leukocytes and CRP on day 4 were higher in patients with poor outcome (lack of disappearance of symptoms and signs of peritonitis during treatment with antibiotics). They reported that CRP > 100 mg/L and dialysate leukocyte count > 350 × 106/L on day 4 indicated poor outcome.Citation25 Fontán et al. found (although statistically not significant) a minor trend to higher peritoneal leukocyte counts in lethal peritonitis.Citation22 In another study by Krishnan et al., for the peritonitis episodes in which the PD effluent cell count was >100 μL for more than 5 days, the nonresolution rate was 45.6%, compared to a 4.2% nonresolution rate when the cell count returned to <100/μL or less in <5 days. They concluded that the number of days the PD effluent cell count remained >100/μL independently predicted the outcome of an episode of peritonitis. Additionally, they found that the number of peritonitis episodes before the episode in question, initial empiric treatment, serum albumin level, total lymphocyte count and initial dialysate WBC count, age, sex, diabetes, previous renal transplantation, and the use of steroids did not affect outcome of peritonitis.Citation17 However, in their study, the data about the time elapsed between the onset of peritonitis symptoms and the time of PD effluent cell count was lacking. In our study, we demonstrated that in PD patients who had been admitted to the hospital ≥24 hours after the onset of symptoms of peritonitis, PD effluent leukocyte (p = 0.04) and neutrophil counts (p = 0.005) were higher and PD effluent lymphomononuclear cell percentage (p = 0.04) was lower than those patients who had been admitted within the first 24 hours.
We conclude that admitting to the hospital within the first 24 hours of peritonitis symptom onset is of vital importance as far as the course of peritonitis is concerned because delayed hospital admission for ≥24 hours is associated with higher PD effluent leukocyte and neutrophil counts, and increased length of hospital stay.
Declaration of interest:The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this paper.
REFERENCES
- Kent JR, Almond MK. A survey of CAPD peritonitis management and outcomes in North and South Thames NHS regions (U.K.): Support for the ISPD guidelines. International Society for Peritoneal Dialysis. Perit Dial Int. 2000;20:301–305.
- Hurst SM, Wilkinson TS, McLoughlin RM, IL-6 and its soluble receptor orchestrate a temporal switch in the pattern of leukocyte recruitment seen during acute inflammation. Immunity. 2001;14:705–714.
- Enriquez J, Klinger J, Arturo JA, Delgado M, Tobar C, Mosquera M. Peritonitis in continuous ambulatory peritoneal dialysis: Cytokines in peritoneal fluid and blood. Adv Perit Dial. 2002;18:177–183.
- Shetty H, Gokal R. Treatment of infections in peritoneal dialysis. Contrib Nephrol. 2003;140:187–194.
- Diaz-Buxo JA. What is the role of automated peritoneal dialysis and continuous flow peritoneal dialysis? Contrib Nephrol. 2003;140:264–271.
- McLoughlin R. Resolving peritoneal inflammation: Flicking the right “switches”. Perit Dial Int. 2005;25:223–229.
- Henderson RB, Hobbs JA, Mathies M, Hogg N. Rapid recruitment of inflammatory monocytes is independent of neutrophil migration. Blood. 2003;102:328–335.
- Tzamaloukas AH. What affects the outcome of peritoneal dialysis? Going beyond the microbial etiology. Perit Dial Int. 2002;22:563–565.
- Glik A, Douvdevani A. T lymphocytes: The “cellular” arm of acquired immunity in the peritoneum. Perit Dial Int. 2006;26:438–448.
- Continuous ambulatory peritoneal dialysis (CAPD) peritonitis treatment recommendations: 1989 update. The Ad Hoc Advisory Committee on Peritonitis Management. Perit Dial Int. 1989;9:247–256.
- Troidle L, Finkelstein F. Treatment and outcome of CPD-associated peritonitis. Ann Clin Microbiol Antimicrob. 2006;5:6.
- Piraino B, Bailie GR, Bernardini J, ISPD Ad Hoc Advisory Committee. Peritoneal dialysis-related infections recommendations: 2005 update. Perit Dial Int. 2005;25:107–131.
- Glik A, Mazar J, Rogachev B, Zlotnik M, Douvdevani A. CD40 ligand expression correlates with resolution of peritonitis and mononuclear cell recruitment. Perit Dial Int. 2005;25:240–247.
- Zelenitsky S, Barns L, Findlay I, Analysis of microbiological trends in peritoneal dialysis-related peritonitis from 1991 to 1998. Am J Kidney Dis. 2000;36:1009–1013.
- Korbet SM, Vonesh EF, Firanek CA. Peritonitis in an urban peritoneal dialysis program: An analysis of infecting pathogens. Am J Kidney Dis. 1995;26:47–53.
- Bernardini J, Holley JL, Johnston JR, Perlmutter JA, Piraino B. An analysis of ten-year trends in infections in adults on continuous ambulatory peritoneal dialysis (CAPD). Clin Nephrol. 1991;36:29–34.
- Krishnan M, Thodis E, Ikonomopoulos D, Predictors of outcome following bacterial peritonitis in peritoneal dialysis. Perit Dial Int. 2002;22:573–581.
- Kim GC, Korbet SM. Polymicrobial peritonitis in continuous ambulatory peritoneal dialysis patients. Am J Kidney Dis. 2000;36:1000–1008.
- Bunke M, Brier ME, Golper TA. Culture-negative CAPD peritonitis: The Network 9 Study. Adv Perit Dial. 1994;10:174–178.
- Troidle L, Majahan A, Gorban-Breannan N, Kliger AS, Finkelstein FO. C-reactive protein, peritonitis and outcome. Perit Dial Int. 2001;21(Suppl. 1):S45.
- Troidle L, Gorban-Brennan N, Kliger A, Finkelstein FO. Continuous peritoneal dialysis-associated peritonitis: A review and current concepts. Semin Dial. 2003;16:428–437.
- Perez Fontan M, Rodriguez-Carmona A, Garcia-Naveiro R, Rosales M, Villaverde P, Valdes F. Peritonitis-related mortality in patients undergoing chronic peritoneal dialysis. Perit Dial Int. 2005;25:274–284.
- Cueto-Manzano AM, Quintana-Pina E, Correa-Rotter R. Long-term CAPD survival and analysis of mortality risk factors: 12-year experience of a single Mexican center. Perit Dial Int. 2001;21:148–153.
- Szeto CC. Peritonitis-cells, cytokines, and local defense. Hong Kong J Nephrol. 2002;4:63–64.
- Martikainen TA, Ekstrand AV, Honkanen EO, Teppo AM, Gronhagen-Riska C. Dialysate leukocytes, sICAM-1, hyaluronan and IL-6: Predictors of outcome of peritonitis? Blood Purif. 2004;22:360–366.
