Abstract
During acute kidney injury (AKI), lowered glomerular filtration rate (GFR) is believed to be consequent to reduced renal plasma flow (RPF). We aimed to systematically evaluate the evidence for such an association. Using specific search terms, we systematically interrogated the Pub Med electronic reference database for studies of human AKI where renal plasma or blood flow and GFR were measured; older articles were then identified by screening bibliographies of retrieved reports. We identified 22 articles describing 250 patients (203 native kidney, 47 in renal allograft). Of these studies, 8 articles (110 patients) estimated effective renal plasma flow (ERPF) by clearance techniques and 14 articles (140 patients) estimated true renal plasma flow (TRPF). Mean RPF was 272 mL/min (95% CI 213–331) and GFR 13.9 mL/min (9.9–17.9). Mean TRPF was significantly greater than mean ERPF (344 vs. 180, p = 0.004) despite lower mean GFR (8.8 vs. 20.4, p = 0.002). There was no significant association between RPF and GFR between studies. Eleven studies presented individual patient data (76 patients: 49 TRPF, 27 ERPF); here, individual patient ERPF was associated with GFR (r2 = 0.52), but TRPF was not. During AKI in man, there is only a limited association between ERPF and GFR, and no detectable association between TRPF and GFR.
INTRODUCTION
Acute kidney injury (AKI) is a common and serious complication of critical illness, affecting around 20% of patients admitted to hospitalCitation1 and over 50% of those admitted to intensive care units.Citation2 Of these, approximately 5% require renal replacement therapy.Citation3 The crude mortality of AKI has changed little over in the last 40 yearsCitation4; however, its epidemiology has altered and sepsis is now a much more common associated condition, occurring in over 50% of cases.Citation5,Citation6 Despite its clinical significance, the pathogenesis of AKI in man remains poorly understood.
AKI is characterized by the sudden and sustained loss of glomerular filtration rate (GFR) with accumulation of nitrogenous waste products. Current descriptions of the pathophysiology of AKI continue to emphasize reduction in renal blood flow (RBF) and consequent renal ischemia as the cause of loss of GFR.Citation6–8 However, surprisingly little clinical evidence is available to support the causative link between reduced perfusion (RPF, renal plasma flow) and reduced function (GFR) in common clinical situations such as sepsis.Citation9 A previous systematic literature review examined reports of RBF measurement during AKI in man and found that while it is, on average, reduced, there was with considerable heterogeneity in measurements within and between studies,Citation10 questioning the nature of the association between reduction in RBF and acute renal impairment. However only a proportion of these studies reported a reliable measure of GFR, precluding analysis of the association between RBF and the severity of AKI. Accordingly we have gone on to identify those studies where both GFR and renal blood or plasma flow have been measured to assess the relationship between these parameters in patients with AKI. We hypothesized that, in keeping with accepted paradigms, there would be a significant correlation between published measurements of RPF and GFR during AKI, confirming the pivotal role of reduced perfusion in the pathogenesis of renal dysfunction.
METHODS
We applied new inclusion criteria: studies of two or more humans with AKI including a numerical estimate of both renal blood or plasma flow and GFR () to our previous systematic literature search for reports of RBF during AKI in man.Citation10 This comprised a Pub Med database search identifying articles indexed under the MESH categories: “Kidney failure, Acute” and “Renal Circulation,” limited to those involving humans and published in the English language, a free text search for references to “acute renal failure” or “acute kidney failure” and “renal blood flow” or “renal plasma flow” in title or abstract and screening of the bibliographies of retrieved publications for older articles also meeting the inclusion criteria. A number of publications reported RBF in kidney transplants with delayed function; clinically this is considered a form of post-ischemic AKI and provides a useful human model of the condition; such articles were thus included as relevant to this review.
TABLE 1. Inclusion criteria
For the purposes of this review, AKI was defined () as an acute worsening of renal excretory function in the absence of documented intrinsic renal disease or outflow tract obstruction and in the presence of a recognized acute renal insult (shock, sepsis, trauma, surgery, toxin, pigment). Excretory failure was defined by a measured GFR of <37.5 mL/min, representing a >50% decrease from a normal GFR of >75 mL/min, thus identifying populations meeting RIFLE I or F criteria by the modern consensus definitionCitation11; descriptions of patients with anuria were taken to represent GFR ≈ 0 and were included in the analysis. The >50% decline in GFR defining RIFLE I was chosen to identify a patient population with clinically overt AKI in the context of acute illness.
Conditions and results of individual studies were tabulated. Where RBF was measured more than once, values were recorded when renal dysfunction was maximal. RPF was the most commonly reported measure of renal perfusion. Accordingly, measurements of RBF were standardized to that of plasma flow to allow comparison between publications and calculation of filtration fraction (FF). In order to make these comparisons, we arbitrarily assumed, in the absence of specific data, a hematocrit of 0.3, and a total renal mass of 300 g, allowing conversion of RBF and RBF per gram of renal tissue to RPF. For single kidney RPF from renal transplants values for RPF were doubled to allow comparison to total (two kidneys) RPF. FF was calculated as GFR divided by RPF.
Mean and standard deviation in RPF were obtained from the retrieved publications or calculated from individually reported patient data. Data are reported as means and 95% confidence interval of the mean. Clearance methods of quantifying RBF measuring an effective renal plasma flow (ERPF) are at theoretical disadvantage compared to measurements of true renal plasma flow (TRPF) by non-clearance methods or clearance corrected for renal extraction. Thus, we compared studies estimating ERPF and those measuring TRPF during AKI. Means were compared using independent two-sample t-tests. ERPF and GFR and TRPF and GFR were displayed on an x–y plot and correlation compared using linear regression analysis; Pearson r2 values calculated and slopes were reported with 95% confidence intervals (Graph Pad Prism, GraphPad Software, La Jolla, CA, USA).
Comparison of mean values from different studies will only reveal correlation between these variables if the different studies examine distinct patient populations. However, correlations existing within individual studies may be entirely masked. This may be particularly the case when studies examine similar patient populations with differing methodology. Given these concerns, we also similarly analyzed pooled individual data from all studies where it was available and compared measurements of ERPF and TRPF separately.
Finally, some studies reported RPF and GFR after recovery of renal function in a small number of patients. Recovery of renal function in individual patients was presented on an x–y plot and mean changes in RPF, GFR, and FF during renal recovery were also calculated.
RESULTS
Using our search strategy, we identified 22 relevant publicationsCitation12–33 including 203 individuals with native AKI and 47 cases involving a renal allograft (). Eight studies (110 patients) measured ERPF and 14 TRPF (140 patients).
TABLE 2. Studies of AKI in man reporting RBF and GFR
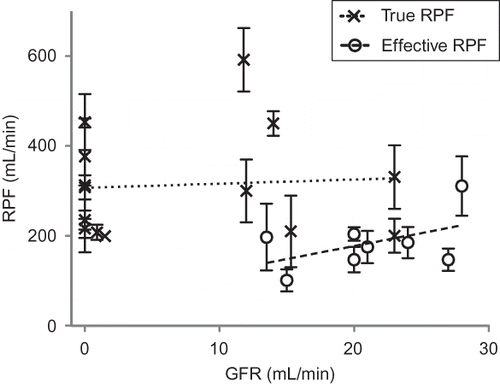
Overall mean RPF during AKI was 272 mL/min (213–331) and GFR 13.9 mL/min (9.9–17.9). However, measurement methodology affected estimates of RPF. Thus, mean TRPF was 344 mL/min, twice the value of ERPF at 180 mL/min (p = 0.004). Paradoxically, however, GFR was lower in studies measuring TRPF (8.8 vs. 20.4 mL/min; p = 0.002). Mean FF was 0.067 (0.044–0.090). It was 0.027 (0.009–0.044) for TRPF and 0.12 (0.10–0.14) for ERPF (p < 0.0001).
Mean RPF and GFR between studies were plotted as an x–y scatter plot (). On linear regression analysis, neither ERPF nor TRPF correlated with GFR (r2 = 0.24 and r2 = 0.005, respectively). Slopes of the regression lines were not significantly different from zero.
FIGURE 1. RPF versus GFR in 22 studies of human with AKI. Bars show standard error of mean RPF. Linear regression lines for ERPF and true RPF are shown (r2 = 0.24 and 0.005, respectively).
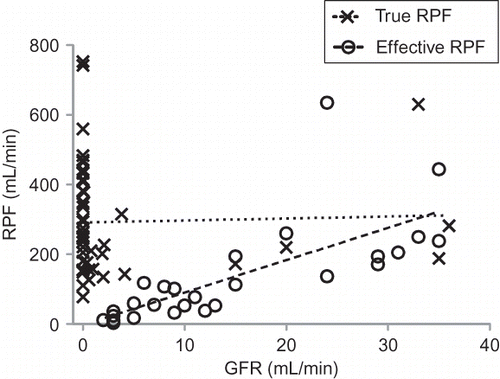
Eleven studiesCitation13,Citation15,Citation16,Citation19–21,Citation23,Citation27–29,Citation33 reported individual data in 76 patients (49 TRPF, 27 ERPF). In these studies, mean TRPF was 293 (249–337) mL/min with a mean GFR of 3.2 (−0.15 to 6.5) mL/min, and a FF of 0.01. Mean ERPF was 135 (80–189) mL/min with a mean GFR of 14.8 (10.6–19.0) mL/min, and a FF of 0.11. Individual patient RPF and GFR was plotted on as an x–y scatter plot (). On linear regression analysis, TRPF did not correlate with GFR (r2 = 0.001); however ERPF showed a modest positive correlation with GFR (r2 = 0.52).
FIGURE 2. RPF versus GFR in 76 patients with individual data reported. Linear regression line for true and effective RPF are shown (r2 = 0.001 and 0.52, respectively, slope for ERPF significantly nonzero p < 0.0001). Selective underestimation of ERPF during more severe renal dysfunction is suggested.
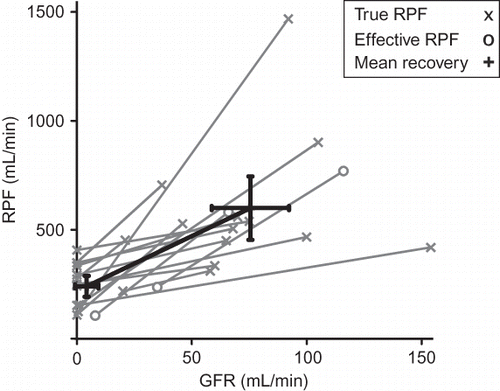
Renal recovery data were available in 15 patients from six studiesCitation13,Citation15,Citation16,Citation21,Citation28,Citation33 (). For these patients, mean RPF was 241 mL/min (193–290) and GFR was 4.3 mL/min (−0.8 to 9.4) recovering to a mean RPF of 601 mL/min (456–746) and a GFR of 76 mL/min (59–92). FF increased from 0.02 to 0.14 with recovery of renal function.
DISCUSSION
Using a systematic strategy, we identified 22 publications reporting renal blood or plasma flow and GFR during AKI in man spanning over half a century. In this setting, RPF was lower than normal but the technique of measurement significantly affected estimates of RPF altering its estimated value by close to 100%. Furthermore, in contradiction to our hypothesis, the reduction in GFR was disproportionately greater than that in TRPF and, in these patients, there was no correlation between TRPF and GFR.
Our findings of reduced RPF in AKI are consistent with previous reports in manCitation34,Citation35; however, they are not consistent with the far greater reduction in RBF required to induce AKI in some experimental models,Citation36–38 highlighting the uncertainty surrounding to what extent decreased perfusion contributes to the development of AKI. This uncertainty is further compounded by concerns about our ability to measure RPF accurately in man as demonstrated by the discrepancy between values obtained with different techniques in our systematic review. Specifically, ERPF is usually measured by the renal clearance of para-aminohippurate (PAH), a marker that is normally almost totally removed from the renal circulation on first pass. During oliguria and AKI, PAH clearance greatly underestimates RPF.Citation39 This is because impairment of tubular uptake and secretion of PAH occurs during tubular injury,Citation40 which in turn reduces renal clearance of PAH to between 5 and 50% of true RPF during AKICitation13,Citation14,Citation27 and by 50% in septic shock even in the absence of a fall in GFR.Citation41,Citation42 These technical limitations probably account for the difference between TRPF and ERPF seen in our study. They are also likely to influence estimates of the relationship between RPF and GFR by artificially increasing it. In keeping with this notion, analysis of data from individual patients in our study revealed a modest but significant positive correlation between measurements of ERPF and GFR, but no such relationship between TRPF and GFR. As ERPF underestimates true RPF in a manner related to the severity of renal tubular injury, it is unsurprising that ERPF should correlate with GFR during AKI even when TRPF does not. In addition, studies using ERPF also differ in context from direct measurements of RPF. ERPF measurements require urine output and have been used historically in patients earlier in the course of AKI, while more complex direct methods (TRPF) have been employed in established AKI often in the context of anuria (see ). These contextual differences between RPF estimates preclude drawing definitive conclusions about renal perfusion during the course of AKI in man and affect assessments of the relationship between perfusion and GFR. Nonetheless, in their aggregate, all of the above observations do not support our hypothesis.
In our systematic review, we found no correlation between GFR and TRPF measurements and only a very weak correlation between GFR and ERPF in patients with AKI. This contrasts with physiological responses to the restriction of RBF in animal models; their diminished renal perfusion is followed by reduction in downstream afferent arteriolar resistance and Angiotensin-II-mediated increase in efferent arteriolar resistanceCitation43 which acts to maintain FF and defend GFR in the face of decreased renal perfusion pressure.Citation44 In these circumstances approximately 4- to 5-fold reduction in RPF was accompanied by an equivalent fall in GFRCitation45 and GFR recovered linearly with RPF as systemic blood pressure increased.Citation45,Citation46 Conversely, during established AKI, where RPF was only moderately reduced in comparison to the decrease in GFR, it appears that autoregulatory mechanisms that maintain coupling between RPF and GFR may either be inoperative or insufficient or both. Consequently, therapeutic strategies aimed at restoring RPF might be ineffective in reversing AKI. These observations were strengthened by analysis of individual patient data and indirectly supported by analysis of 15 patients with measurements of RPF and GFR during both AKI and recovery (), which demonstrate a proportionately greater rise in GFR than RPF during recovery reflecting a large rise in FF from very low levels during AKI. The aggregate of these findings suggest that factors beyond an absolute reduction in RPF are, at least in part, responsible for the loss of GFR during AKI.
There are several possible explanations for this uncoupling between RBF and GFR. These include raised Bowman's space pressure secondary to tubular obstruction and/or failure of active reabsorption of ultrafiltrate; back-leak of tubular ultrafiltrate into the interstitium and circulation; tubulo-glomerular feedback-induced afferent arteriolar vasoconstrictionCitation12,Citation47; or decreased efferent arteriolar tone.Citation48 Our study cannot clarify what mechanisms explain the uncoupling of GFR from RBF, but by demonstrating this phenomenon, it can help focus future research into this aspect of the pathophysiology of AKI in man.
This systematic review has strengths and limitations. To our knowledge, it represents the first systematic study of the relationship between RBF and GFR in man during AKI. Earlier reviews of the hemodynamics of AKICitation34,Citation35 only described a fraction of the studies available in a nonsystematic fashion. By demonstrating the lack of a relationship between TRPF and GFR, it challenges the validity, experimental basis for, and scientific robustness of the prevailing paradigm that GFR is lost in AKI in man because of decreased RBF. However, given the diverse nature of the literature, there are some significant limitations. In 22 publications spanning a 60-year period, there was considerable heterogeneity in patient populations, cause of AKI, methodology for estimation of RPF, and treatment provided. However, this observation itself further reinforces the overall finding of our study that the data supporting current paradigms are weak. Comparing the results of these studies required the assumption of a hematocrit of 30% to allow calculation of RPF from RBF. As anemia is extremely frequent in AKICitation49 and critical illnessCitation50 this approach seems justified. However, even if inaccurate, assumptions of other realistic values for such patients (hematocrit between 25 and 35%) would not materially alter our findings. Furthermore, the absence of such specific information in the available literature once again lends support to our findings by showing further deficiencies in the available data. Comparison of mean values between studies may fail to detect statistical correlations between variables within individual studies. In light of this concern, analysis of individual patient was undertaken, but these data were only available in a small number of older studies. By examining patients with falls in GFR > 50% we may have focused on too narrow a part of the spectrum of AKI. However, the severity of renal dysfunction in the critically ill is often significantly underestimated.Citation51 Consequently, to examine the relationship between RPF and GFR at less severe levels of acute renal dysfunction would be difficult and lack relevance to the problem of clinically overt AKI in the ICU. In any case no such data are available.
CONCLUSIONS
Contrary to our expectations, in AKI in man, published studies do not show a correlation between TRPF and GFR, implying uncoupling between perfusion (TRPF) and function (GFR), such that for a given decrease in decreased perfusion, there is an unpredictable and much greater loss in function. These observations are important in highlighting the need for further measurements of why and how GFR decreases in AKI as, in patients with clinically significant AKI, severity of reduction in GFR does not seem to reliably correlate with the degree of reduction in RPF. This information is vital to our understanding of the pathogenesis of this condition and our ability to develop targeted therapeutic strategies.
Acknowledgment
Source of funding: Austin Health ICU Research Fund.
REFERENCES
- Uchino S, Bellomo R, Goldsmith D, Bates S, Ronco C. An assessment of the RIFLE criteria for acute renal failure in hospitalized patients. Crit Care Med. 2006;34:1913–1917.
- Hoste EA, Clermont G, Kersten A, RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: A cohort analysis. Crit Care. 2006;10:R73.
- Uchino S, Kellum JA, Bellomo R, Acute renal failure in critically ill patients: A multinational, multicenter study. JAMA. 2005;294:813–818.
- Lameire N, Van Biesen W, Vanholder R. The changing epidemiology of acute renal failure. Nat Clin Pract Nephrol. 2006;2:364–377.
- de Mendonça A, Vincent JL, Suter PM, Acute renal failure in the ICU: Risk factors and outcome evaluated by the SOFA score. Intensive Care Med. 2000;26:915–921.
- Schrier RW, Wang W. Acute renal failure and sepsis. N Engl J Med. 2004;351:159–169.
- Badr KF. Sepsis-associated renal vasoconstriction: Potential targets for future therapy. Am J Kidney Dis. 1992;20:207–213.
- De Vriese AS, Bourgeois M. Pharmacologic treatment of acute renal failure in sepsis. Curr Opin Crit Care. 2003;9:474–480.
- Wan L, Bagshaw SM, Langenberg C, Saotome T, May C, Bellomo R. Pathophysiology of septic acute kidney injury: What do we really know? Crit Care Med. 2008;36:S198–S203.
- Prowle JR, Ishikawa K, May CN, Bellomo R. Renal blood flow during acute renal failure in man. Blood Purif. 2009;28: 216–225.
- Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P, Acute Dialysis Quality Initiative workgroup. Acute renal failure – definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–R212.
- Alejandro V, Scandling JD, Sibley RK, Mechanisms of filtration failure during postischemic injury of the human kidney. A study of the reperfused renal allograft. J Clin Invest. 1995;95:820–831.
- Brun C, Crone C, Davidsen HG, Renal blood flow in anuric human subject determined by use of radioactive Krypton 85. Proc Soc Exp Biol Med. 1955;89:687–690.
- Corrigan G, Ramaswamy D, Kwon O, PAH extraction and estimation of plasma flow in human postischemic acute renal failure. Am J Physiol. 1999;277:F312–F318.
- Epstein M, Berk DP, Hollenberg NK, Renal failure in the patient with cirrhosis. The role of active vasoconstriction. Am J Med. 1970;49:175–185.
- Hollenberg NK, Epstein M, Rosen SM, Basch RI, Oken DE, Merrill JP. Acute oliguric renal failure in man: Evidence for preferential renal cortical ischemia. Medicine (Baltimore). 1968;47:455–474.
- Hollenberg NK, Adams DF, Oken DE, Abrams HL, Merrill JP. Acute renal failure due to nephrotoxins. N Engl J Med. 1970;282:1329–1334.
- Ilic S, Rajic M, Vlajkovic M, Bogicevic M, Stefanovic V. The predictive value of 131I-hippurate clearance in the prognosis of acute renal failure. Ren Fail. 2000;22:581–589.
- Ladefoged J, Winkler K. Hemodynamics in acute renal failure. The effect of hypotension induced by dihydralazine on renal blood flow, mean circulation time for plasma, and renal vascular volume in patients with acute oliguric renal failure. Scand J Clin Lab Invest. 1970;26:83–87.
- Ladefoged J. Increase in renal blood flow in acute renal failure following intra-arterial infusion of acetylcholine. Scand J Clin Lab Invest. 1977;37:709–712.
- Lauson HD, Bradley SE, Cournand A, Andrews VV. The renal circulation in shock. J Clin Invest. 1944;23:381–402.
- Lenz K, Hörtnagl H, Druml W, Ornipressin in the treatment of functional renal failure in decompensated liver cirrhosis. Effects on renal hemodynamics and atrial natriuretic factor. Gastroenterology. 1991;101:1060–1067.
- Moyer JH, Morris G, Beazley HL. Renal hemodynamic response to vasopressor agents in the treatment of shock. Circulation. 1955;12:96–107.
- Myers BD, Miller DC, Mehigan JT, Nature of the renal injury following total renal ischemia in man. J Clin Invest. 1984;73:329–341.
- Pedersen F, Ladefoged J. Renal hemodynamics in acute renal failure in man measured by intra-arterial injection. External counting technique with xenon-133 and I-131-albumin. Scand J Urol Nephrol. 1973;7:187–195.
- Ramaswamy D, Corrigan G, Polhemus C, Maintenance and recovery stages of postischemic acute renal failure in humans. Am J Physiol Renal Physiol. 2002;282:F271–F280.
- Reubi FC, Gossweiler N, Gürtler R. Renal circulation in man studied by means of a dye-dilution method. Circulation. 1966;33:426–442.
- Reubi FC, Vorburger C, Tuckman J. Renal distribution volumes of indocyanine green, (51 Cr) EDTA, and 24 Na in man during acute renal failure after shock. Implications for the pathogenesis of anuria. J Clin Invest. 1973;52:223–235.
- Shalmi CL, Dutcher JP, Feinfeld DA, Acute renal dysfunction during interleukin-2 treatment: Suggestion of an intrinsic renal lesion. J Clin Oncol. 1990;8:1839–1846.
- Swärd K, Valson F, Ricksten SE. Long-term infusion of atrial natriuretic peptide (ANP) improves renal blood flow and glomerular filtration rate in clinical acute renal failure. Acta Anaesthesiol Scand. 2001;45:536–542.
- Valsson F, Ricksten SE, Hedner T, Zäll S, William-Olsson EB, Lundin S. Effects of atrial natriuretic peptide on renal function after cardiac surgery and in cyclosporine-treated heart transplant recipients. J Cardiothorac Vasc Anesth. 1994;8:425–430.
- Valsson F, Ricksten SE, Hedner T, Lundin S. Effects of atrial natriuretic peptide on acute renal impairment in patients with heart failure after cardiac surgery. Intensive Care Med. 1996;22:230–236.
- Walker JG, Silva H, Lawson TR, Ryder JA, Shaldon S. Renal blood flow in acute renal failure measured by renal arterial infusion of indocyanine green. Proc Soc Exp Biol Med. 1963;112:932–935.
- Reubi FC. The pathogenesis of anuria following shock. Kidney Int. 1974;5:106–110.
- Myers BD, Moran SM. Hemodynamically mediated acute renal failure. N Engl J Med. 1986;314:97–105.
- Phillips RA, Dole VP, Hamilton PB, Emerson K, Archibald RM, Van Slyke DD. Effects of acute hemorrhagic and traumatic shock on renal function of dogs. Am J Physiol. 1946;122:765.
- Badenoch AW, Darmady EM. The effects of temporary occlusion of the renal artery in rabbits and its relationship to traumatic uraemia. J Pathol Bacteriol. 1947;59:79.
- Zager RA. Partial aortic ligation: A hypoperfusion model of ischemic acute renal failure and a comparison with renal artery occlusion. J Lab Clin Med. 1987;110:396–405.
- Selkurt EE. Renal blood flow and renal clearance during hemorrhagic shock. Am J Physiol. 1946;145:699.
- Kwon O, Corrigan G, Myers BD, Sodium reabsorption and distribution of Na+/K+-ATPase during postischemic injury to the renal allograft. Kidney Int. 1999;55:963–975.
- Lucas CE, Rector FE, Werner M, Rosenberg IK. Altered renal homeostasis with acute sepsis. Clinical significance. Arch Surg. 1973;106:444–449.
- Rector F, Goyal S, Rosenberg IK, Lucas CE. Sepsis: A mechanism for vasodilatation in the kidney. Ann Surg. 1973;178:222–226.
- Anderson WP, Johnston CI, Korner PI. Acute renal hemodynamic and renin–angiotensin system responses to graded renal artery stenosis in the dog. J Physiol. 1979;287:231–245.
- Schweitzer G, Gertz KH. Single-nephron hemodynamics in one-kidney one-clip hypertension in the rat. Ren Physiol. 1984;7:46–53.
- DeForrest JM, Davis JO, Freeman RH, Watkins BE, Stephens GA. Separate renal function studies in conscious dogs with renovascular hypertension. Am J Physiol. 1978;235:F310–F316.
- Gabbai FB, Gushwa LC, Peterson OW, Wilson CB, Blantz RC. Analysis of renal function in the two-kidney Goldblatt model. Am J Physiol. 1987;252:F131–F137.
- Sutton TA, Molitoris BA. Mechanisms of cellular injury in ischemic acute renal failure. Semin Nephrol. 1998;18:490–497.
- Langenberg C, Wan L, Egi M, May CN, Bellomo R. Renal blood flow and function during recovery from experimental septic acute kidney injury. Intensive Care Med. 2007;33:1614–1618.
- Hales M, Solez K, Kjellstrand C. The anemia of acute renal failure: Association with oliguria and elevated blood urea. Ren Fail. 1994;16:125–131.
- Corwin HL, Gettinger A, Pearl RG, The CRIT study: Anemia and blood transfusion in the critically ill–current clinical practice in the United States. Crit Care Med. 2004;32:39–52.
- Clark WR, Mueller BA, Kraus MA, Macias WL. Quantification of creatinine kinetic parameters in patients with acute renal failure. Kidney Int. 1998;54:554–560.