Abstract
Introduction: In this study, we aimed to analyze the effects of once- or thrice-weekly mupirocin application on peritonitis, exit-site infection (ESI), and antibiotic resistance with mupirocin. Patients and methods: By 2000 mupirocin began to be applied once a week to 33 patients who previously did not use mupirocin at the exit site. By the beginning of 2002, the patients were assigned to two groups. In group I patients continued to apply mupirocin once a week. In group II patients began to apply mupirocin to the exit site three times weekly and we began to obtain cultures from the nares, inguinal area, axillae, and the exit site. Results: A total of 28 episodes of ESI and 41 episodes of peritonitis were seen in 33 patients prior to mupirocin treatment, while a total of 14 episodes of ESI and 34 episodes of peritonitis were observed in all groups of patients who used mupirocin. In a subgroup analysis, 13 episodes of peritonitis and 7 episodes of ESI were determined in group I, while 6 episodes of peritonitis and 1 episode of ESI were determined in group II. Staphylococcus aureus reproduction rate and mupirocin resistance were 2.11 and 0.2%, respectively. Coagulase-negative staphylococcus reproduction rate was 70.56% (MuR: 59.87% and MeR: 33.7%) and 72.6% (MuR: 64.7% and MeR: 33.3%) in groups I and II, respectively. Conclusion: Mupirocin application at the exit sites reduces peritonitis and ESI to a considerable amount, and thrice-weekly application of mupirocin seems to be more efficient compared to once-weekly application.
INTRODUCTION
Peritoneal dialysis (PD) holds an important place in the treatment of end-stage renal disease (ESRD). Despite the advancement of technology, peritonitis, exit-site infections (ESI), and technical failures remain as the leading causes of morbidity in PD patients.Citation1,Citation2 However, the infection rates have decreased considerably during the last decade by the efficient control of ESI.
ESIs appear mostly due to Staphylococcus aureus and coagulase-negative staphylococcus (CNS). The presence of nasal or exit-site carriage or both is crucial in the development of ESI. The prevention of this carriage is highly efficient in the reduction of the infection rate.Citation3 As it was shown in the previous studies, the use of prophylactic antibiotics at the exit site or orally (neomycin sulfate, mupirocin, rifampin, TMP-SMX, ciprofloxacin) reduce S. aureus colonization and catheter-related infection rate.Citation4–7
In the recent studies, it has been shown that application of mupirocin to the exit site considerably reduces ESI and peritonitis rate.Citation8,Citation9 Currently, the most popular regimen for mupirocin is to apply it at the exit site once a day, 3–5 times a week.Citation10–12 However, 3–5 times a week application of mupirocin may decrease patient compliance and may increase antibiotic resistance.Citation13 With the emerging threat of mupirocin resistance with prolonged application, it becomes imperative to weigh the risks and benefits of the universal use of mupirocin.
In this study, we aimed to determine the potential effectiveness of the application of once- or thrice-weekly mupirocin cream at the catheter exit site in preventing ESI and peritonitis. In addition, the other objective of our study was to determine the effects of long-term mupirocin application on MuR and MeR in S. aureus and also CNS in PD patients.
PATIENTS AND METHODS
Thirty-three patients (19 men, 14 women; mean age: 56 ± 7.6 years) undergoing PD treatment were included in this prospective study. By 2000 mupirocin in the form of 2% ointment (Bactroban®, GlaxoSmithKline, Istanbul, Turkey) began to be applied once a week to all 33 patients who previously did not apply mupirocin at the exit site. By the beginning of 2002, the patients were assigned to two groups. In group I (17 patients: 10 men, 7 women; mean age: 58 ± 7.99 years) patients continued to apply mupirocin once a week. In group II (16 patients: 9 men, 7 women; mean age: 54 ± 8 years) patients began to apply mupirocin to the exit site three times weekly. As the obtainment of culture was not planned at the beginning, we began to obtain cultures from the nares, inguinal area, axillae, and exit site only by 2002 ().
Patients were evaluated for evidence of ESI or peritonitis during each monthly outpatient clinic. ESI was diagnosed according to the criteria defined by Twardovski and was defined as erythema, purulent drainage, and sensitivity at the skin surface and catheter–skin interface. The diagnosis of peritonitis, on the other hand, was made upon the determination of two of the three criteria: cloudy peritoneal effluent, leukocyte exceeding 100/mm3 (polymorphonuclear leukocytes exceeding 50%), abdominal pain, and bacterial growth in the peritoneal effluent culture.Citation14 The samples were incubated on a blood agar plate for 18–24 hours at 37°C and isolates were detected. The colonizations were observed in terms of mupirocin and methicillin resistance on Mueller Hinton agar with disk diffusion method. An inhibition zone larger than 18 mm in CNS and larger than 13 mm in S. aureus was considered as oxacillin-sensitive and an inhibition zone larger than 14 mm was considered as mupirocin-sensitive in both CNS and S. aureus.
In pre-mupirocin treatment period, data for ESIs, peritonitis attacks, and the microorganisms grown in culture of the patients were collected retrospectively.
STATISTICS
The demographic group data were compared by using nonparametric Mann–Whitney U-test. Comparisons between groups were performed by chi-square and Fisher exact tests for categorical data. p < 0.05 was defined as statistically significant.
RESULTS
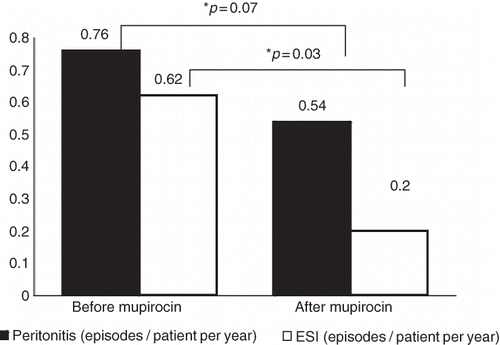
Patients were followed up for 3.0 ± 1.9 years prior to mupirocin treatment. A total of 28 episodes of ESI (0.62 episodes/patient per year) and 41 episodes of peritonitis (0.76 episodes/patient per year) were seen in 33 patients prior to mupirocin treatment, while a total of 14 episodes of ESI (0.20 episodes/patient per year) and 34 episodes of peritonitis (0.54 episodes/patient per year) were observed in all groups of patients who used mupirocin 1 or 3 times a week for 5 years; the decrease was determined to be 28% in peritonitis (p = 0.07) and 64% in ESI (p = 0.03) ().
FIGURE 2. The effect of mupirocin application on development of peritonitis. *As compared to period prior to mupirocin application.
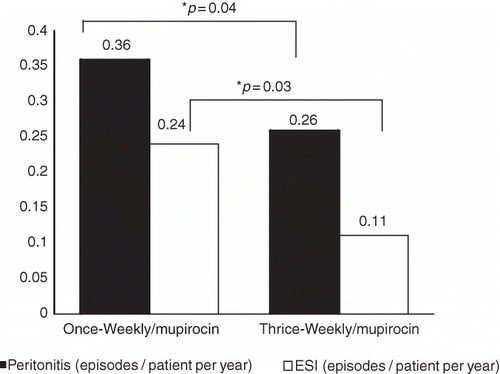
In a subgroup analysis, patients were divided into two groups as those who used mupirocin once-a-week and those who used mupirocin thrice-a-week, and the results of 3 years of follow-up were evaluated. Thirteen episodes of peritonitis (0.36 episodes/patient per year) and seven episodes of ESI (0.26 episodes/patient per year) were determined in group I, while six episodes of peritonitis (0.24 episodes/patient per year) and one episode of ESI (0.11 episodes/patient per year) were determined in group II. It was determined that the rate of peritonitis was 56% (p = 0.04) and of ESI was 92% (p = 0.03) lower in group II when compared to group I ().
FIGURE 3. Comparison of once-a-week versus thrice-a-week application of mupirocin. *As compared to once-a-week application of mupirocin.
A total of 1852 samples were analyzed between 2002 and 2005. In the 992 samples collected in group I, CNS rate was 70.56% (MuR: 59.87% and MeR: 33.7%) and S. aureus rate was 2.11% (MuR: 0.20%) of the patients, respectively. In group II, 860 samples were analyzed and CNS isolation rate was determined as 72.56% (MuR: 64.7%, MeR: 33.3%) while S. aureus isolation rate was determined as 0.93% while no mupirocin resistance was observed in S. aureus in this group of patients. Methicillin resistance was not observed in S. aureus in both groups ( and ). In group I, S. aureus isolation rate was higher (p > 0.05). It was observed that nasal S. aureus carriage rate was higher in the once-weekly mupirocin application group (1.6%) compared to the thrice-weekly mupirocin application group (0.5%). Additionally, the peritonitis and ESI rates were higher in group I but peritonitis and ESI attributable to S. aureus was not observed in both groups. While gram-negative growth rate was determined as 1.20% in group I and 1.74% in group II, gram-negative infection rate was determined to be lower in group II.
TABLE 1. The results of the 5 years; peritonitis, ESI, microbiological analysis with antibiotic resistance patterns
TABLE 2. Distribution of bacteria grown in swab cultures according to the site of culture
DISCUSSION
In this study, we showed that mupirocin application at the exit site reduces peritonitis and ESI in PD patients. Three to five times weekly mupirocin application at the exit site is recommended in the literature, and there are few studies on less-frequent mupirocin applications. In our study, a statistically significant reduction in ESI was shown subsequent to once-weekly mupirocin application and better results were obtained after switching to thrice-weekly mupirocin application.
In a randomized controlled study, Bernardini et al. compared empirical cyclic oral rifampin administration with daily mupirocin application at the exit site. Eighty-two PD patients were included in the study and cyclic oral rifampicin was administered to half of the patients (5 days in a period of 3 months) while mupirocin was administered daily to the exit site in the other half. After a follow-up that lasted 1 year in average, a significant reduction in the infection rates of both groups was observed (peritonitis: 33%, ESI: 58%) while no difference between the groups was recorded.Citation5 Adverse effects due to the use of rifampicin were seen in 12% of the patients using the drug, and mupirocin was recommended as an efficient and alternative treatment to the patients who could not tolerate oral use of rifampicin.
Casey et al. conducted a study that included 291 PD patients. Of these, 143 administered mupirocin at the exit site daily and no treatment was applied to the remaining 148 patients. After 1 year, the authors observed a 49% reduction in the rate of ESI and a 31% reduction in the rate of peritonitis.Citation15 Mahajan et al. included 40 patients in a recent study and administered mupirocin at the exit sites of the patients for 1 year and compared the results with the effect of placebo in the control group. After 1 year, the authors observed a 60% reduction in ESI and 55% reduction in peritonitis.Citation9
In our study, we observed a 75% reduction in the rate of ESI and a 50% reduction in the rate of peritonitis even in the once-weekly mupirocin application group at the end of 5 years. We concluded that this may be the result of long-term application (5 years) and treatment compliance.
Thodis et al. compared once-weekly mupirocin application at the exit site (43 patients) to thrice-weekly mupirocin application (27 patients) in PD patients and observed a 91% reduction in the rate of ESI and a 69% reduction in the rate of peritonitis after 1 year and the authors did not determine a significant difference between the two groups.Citation11 In our study, however, the rates of ESI and peritonitis at the end of 3 years were 92 and 56% lower, respectively, in the thrice-weekly mupirocin application group compared to group I. Moreover, the reduction rates of ESI and peritonitis were found to be statistically significant in our study.
The most significant problem to be faced subsequent to long-term application is the development of mupirocin resistance. Mupirocin was first used in 1980 and the first resistant strains were reported in 1987. In many recent studies, MuR S. aureus strains are reported in PD patients.Citation12,Citation13
Annigeri et al. conducted a study including 146 PD patients who applied mupirocin to the exit site 1–4 times per week and at the end of 4 years MuR S. aureus resistance was determined as 3% and no methicillin resistance was found.Citation16 On the other hand, Perez-Fontan et al. determined a MuR S. aureus rate of 0–12.4% in 155 patients followed between 1990 and 2000. Theirs was a long-term study and changes in treatment were made in the study groups. For this reason, differences in mupirocin resistance were determined.Citation17
Lobbedez et al. included 147 PD patients in their study who received mupirocin treatment at the exit site 1–3 times weekly. At the end of 4 years, the authors determined MuR S. aureus growth rate and MeR rate in these patients as 2.7 and 1.4%, respectively.Citation18 In our study, MuR S. aureus rate was 0.20% in group I while no mupirocin resistance was determined in group II. These rates are lower compared to other studies, and we believe that this difference originates from genetic and environmental factors along with patient compliance. MeR was not detected in S. aureus in both groups. Hence, multi-drug resistance strains are not in question in our patient group for this moment.
CNS has an important role in ESI and peritonitis development. In our study, five patients with peritonitis (38%) and three patients with ESI (42%) were determined in group I and four patients with peritonitis and one patient with ESI were determined in group II. Kesli et al. conducted a study, in which CNS growth rate and MeR in the cultures examined at the laboratory were determined as 62 and 66%, respectively.Citation19 The number of studies in the literature MeR of CNS in PD patients is limited. In our study, CNS was investigated in terms of MuR and MeR and in this regard, our study is the first one in this field. CNS was determined as 70.56% (MuR: 59.87%, MeR: 33.7%) in group I and as 72.56% in group II (MuR: 64.7%, MeR: 33.3%) and more comprehensive studies are needed in this field.
In our study, a total of 16 S. aureus isolates were detected in five of the patients in group I while a total of five isolates were detected in three patients in group II. Nasal carriage rate was higher in group I. This was interpreted to be a coincidental result as the patients were randomized according to age and sex. Although the ESI and peritonitis rates were decreased in group I, these were higher compared to group II. Nasal carriage might have an effect on this result, but the lack/absence of S. aureus growth at the exit-site cultures of both groups reduces the likelihood of this hypothesis.
Lim et al. conducted a study including 740 PD patients and the patients who were receiving mupirocin treatment at the exit site were compared to those who were not. The gram-negative peritonitis rates were determined to be lower in the nontreatment group (p < 0.005) while a difference was not determined in ESI in terms of gram-negative factor.Citation20 In our study, a comparison of gram-negative infection risk was made between the groups and gram-negative peritonitis rate was determined to be lower in the thrice-weekly mupirocin group (p < 0.05). Additionally, no fungal infections were detected during our study.
There are limitations to this study. The numbers are small and no power calculations were performed prior to the commencement of the study. As a result, the study might be underpowered.
CONCLUSION
Mupirocin application at the exit site reduces peritonitis and ESI to a considerable amount, and thrice-weekly application of mupirocin seems to be more efficient compared to once-weekly application. Although the symptoms of serious infection forms may not be observed, infection risk attributable to methicillin- and mupirocin-resistant CNS should be kept in mind.
Declaration of interest:The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Keogh AM. Complications in peritoneal dialysis peritonitis and exit site infections. Perit Dial Int. 1996;16:S464–S467.
- Fried LF, Bernardini J, Johnston JR, Piraino B. Peritonitis influences mortality in peritoneal dialysis patients. J Am Soc Nephrol. 1996;7:2176–2182.
- Grupta B, Bernardini J, Piraino B. Peritonitis associated with exit site and tunnel infections. Am J Kidney Dis. 1996;28:415–419.
- Perez-Fontan M, Rosales M, Rodriguez-Carmona A, Treatment of S. aureus nasal carriers in CAPD with mupirocin. Adv Perit Dial. 1992;8:242–245.
- Bernardini J, Piraino B, Holley J, Juhnston JR, Lutes R. A randomized trial of S. aureus prophylaxis in peritoneal dialysis patients: Mupirocin calcium ointment 2% applied to exit site versus cyclic oral rifampin. Am J Kidney Dis. 1996;27:695–700.
- Chu KH, Choy WY, Cheung CC, Prospective study of the efficacy of local application of gentamicin versus mupirocin in the prevention of peritoneal dialysis catheter-related infections. Perit Dial Int. 2008;28:505–508.
- Xu G, Tu W, Xu C. Mupirocin for preventing exit-site infection and peritonitis in patients undergoing peritoneal dialysis. Nephrol Dial Transplant. 2010;25:587–592.
- Ward A, Campoli-Richards DM. Mupirocin. A rewiew of its antibacterial activity, pharmacokinetic properties and therapeutic use. Drugs. 1986;32:425–444.
- Mahajan S, Tiwari S, Kalra V, Effect of local mupirocin application on exit-site infection and peritonitis in an Indian peritoneal dialysis population. Perit Dial Int. 2005;25:473–477.
- Uttley L, Vardhan A, Mahajan S, Smart B, Hutchinson A, Gokal R. Decrease in infections with the introduction of mupirocin cream at the peritoneal dialysis catheter exit-site. J Nephrol. 2004;17:242–245.
- Thodis E, Bhaskaran S, Passadakis P, Bargman JM, Vas SI, Oreopoulos DG. Decrease in Staphylococcus aureus exit-site infections and peritonitis in CAPD patients by local application of mupirocin ointment at catheter exit site. Perit Dial Int. 1998;18:261–270.
- Cavdar C, Saglam F, Sifil A, Effect of once-a-week vs thrice-a-week application of mupirocin on methicillin and mupirocin resistance in peritoneal dialysis patients: Three years of experience. Ren Fail. 2008;30:417–422.
- Rosales M, Perez-Fontan M, Rodriguez-Carmona A, Garcia-Falcon T. Increasing resistance to mupirocin of S. aureus strains isolated from peritoneal dialysis patients and their partners: Long term study. Perit Dial Int. 2001;21:S218.
- Peritoneal Dialysis–Related Infections Recommendations: 2005 Update. Perit Dial Int. 2005;25:107–131.
- Casey M, Taylor J, Clinard P, Application of mupirocin cream at the catheter exit site reduces exit cite infections and peritonitis in peritoneal dialysis patients. Perit Dial Int. 2000;20:566–568.
- Annigeri R, Conly J, Vas S, Emergence of mupirocin–resistant Staphylococcus aureus in chronic peritoneal dialysis patients using mupirocin prophylaxis to prevent exit-site infection. Perit Dial Int. 2001;21:554–559.
- Perez-Fontan M, Rosales M, Rodriguez-Carmona A, Falcon TG, Valdes F. Mupirocin resistance after long-term use Staphylococcus aureus colonization in patients undergoing chronic peritoneal dialysis. Am J Kidney Dis. 2002;39:337–341.
- Lobbedez T, Gardam M, Dedier H, Routine use of mupirocin at the peritoneal catheter exit site and mupirocin resistance: Still low after 7 years. Nephrol Dial Transplant. 2004;19:3140–3143.
- Kesli R, Cander S, Celebi S. Fusidic acid resistance of S. aureus. Med J Kocatepe. 2004;5:33–36.
- Lim CT, Wong KS, Foo MW. The impact of topical mupirocin on peritoneal dialysis infection rates in Singapore General Hospital. Nephrol Dial Transplant. 2005;20:2202–2206.