Abstract
Aim: In this study we examined the effect of oral application of garlic form [garlic oil (GO)] on rats after renal ischemia–reperfusion (I/R) injury. Materials and methods: Forty male Wistar albino rats were divided into four groups: control, sham-operated, I/R, and I/R + GO. GO was diluted in water and administered by oral intubation three times each week for 6 weeks. All rats except sham-operated underwent 45 min of bilateral renal ischemia followed by 6 hr of reperfusion. Blood samples and kidney tissues were harvested from the rats, and then rats were killed. Serum urea, creatinine, and cystatin C levels were determined. Total antioxidant capacity (TAC), catalase (CAT), total oxidant status (TOS), oxidative stress index (OSI), myeloperoxidase (MPO), nitrite oxide (NO), and protein carbonyl (PC) levels in kidney tissue and blood were measured. In addition, kidney tissue histopathology was evaluated. Results: The serum urea, creatinine, and cystatin C levels were significantly higher in I/R group compared to I/R + GO group (p < 0.01). The serum and tissue antioxidant markers (TAC, CAT) were significantly lower in I/R group than I/R + GO group (p < 0.01). The serum oxidant markers (TOS, MPO, NO, and PC) were significantly higher in I/R group than I/R + GO group (p < 0.01). Also oral application of GO was effective in decreasing of tubular necrosis score. Conclusion: Based on the present data, we conclude that increased antioxidants and decreased oxidants modulated by oral application of GO attenuated the renal I/R injury.
INTRODUCTION
Renal ischemia–reperfusion (I/R) injury is a leading cause of acute renal failure and may be due to trauma, sepsis, or renal transplantation. It plays a critical role in pathogenesis of organ transplantation and rejection.Citation1 Despite the growing body of research, the knowledge about the treatment of renal I/R injury remains limited. Renal ischemia initiates a series of events that can ultimately lead to cellular dysfunction and necrosis.Citation2 However, reperfusion can paradoxically create more tissue injury by initiating the complex cellular events which result in renal injury and the concluding death of renal cells due to a combination of apoptosis and necrosis. High levels of oxygen-free radicals, produced on reperfusion, play a critical role in the injury caused by I/R. This process leads to cell damage, and initiation of apoptotic and necrotic cascades.Citation3
Garlic (Allium sativum L.) is an important and widely cultivated plant with both culinary and medicinal uses stemming from its biological activities, which include antibiotic, anticancer, antithrombotic, and lipid-lowering cardiovascular effects. Despite such extensive medicinal use of garlic for centuries, there was little scientific support for its therapeutic and pharmacological properties. However, there has been a recent upsurge of research on the therapeutic potential of garlic, aiming to understand its exact mechanism of action in different clinical situations, so that garlic and its products may have more judicious future applications.Citation4 Garlic in different forms has antioxidant properties. These properties are shown to be due to the existence of compounds such as water-soluble organosulfur compounds, S-allylcysteine and lipid-soluble compounds such as diallyl sulfide. The in vivo and in vitro I/R studies showed that prophylactic administration of aqueous garlic prior to I/R inhibit lipid peroxidation and prevent depletion in glutathione through its compounds that led to functional recovery. Its ability to inhibit neutrophil migration could suppress fibrosis formation. These preventive effects are seen in models that studied organs such as kidney and liver with functional recovery. Organ system-specific activity such as angiotensin-converting, enzyme-inhibiting activity contributes to a cardio-protective and blood pressure-lowering effect.
In the pertinent literature, studies on effect of different garlic forms on the renal I/R injury have been performed by parenteral application. However, garlic is used universally by oral way as a flavoring agent, traditional medicine, and a functional food to enhance physical and mental health. To our knowledge, the study on effect of oral application of garlics on the renal I/R injury has not been performed. In this study we aimed to investigate whether oral administration of garlic oil (GO) protects the kidney against the renal I/R injury in rats.
MATERIAL AND METHODS
Animals and experimental design
Experimental study was performed in forty 3-month-old Wistar albino rats weighing 200–250 g. All animals were maintained under standard conditions and treated in compliance with the National Institutes of Health guidelines. They were housed on a 12-hr dark/light cycles schedule with lights on at 06:00 am. Rats were deprived of food, but not water, for 12 hr before surgery. Food and water were available ad libitum. Experiments were done in Harran University Experimental Research Center, Sanliurfa, Turkey. The rats were randomly assigned to four experimental groups (n = 10): control, sham-operated, I/R (non-treated), and GO-treated I/R.
The abdomen was opened through a midline incision after the abdomen was shaved and disinfected. Thereafter, I/R injury was induced by clamping both renal vascular pedicles for 45 min, followed by 6 hr of reperfusion with saline (2 mL/kg/hr). After finishing the experiment, blood samples were taken and analyzed for tests of renal function. Only laparotomy was performed to the sham group.
Compounds used in this study were purchased from a local herb store at Sanliurfa, Turkey (Garlic oil softgels, Solgar Vitamin and Herb, Leonia, NJ, USA). The treated dose of GO was 200 mg/kg body weight. GO was diluted in water and administered by oral intubation three times each week for 6 weeks. Body weight, and food and water consumption were measured weekly for the feeding period of the diet. At the end of the procedures, rats were killed and renal tissue and blood samples were obtained from all the rats. A part of kidney was stored at −80°C for the analyses of total antioxidant capacity (TAC), catalase (CAT), total oxidant status (TOS), oxidative stress index (OSI), myeloperoxidase (MPO), nitrite oxide (NO), and protein carbonyl (PC) levels. Plasma samples were analyzed for markers of renal impairment. Biochemical analyses for the plasma blood samples – urea, creatinine, and neutrophil count – were analyzed. Blood urea, creatinine, and cystatin C concentrations were estimated by commercially available kits in an autoanalyzer (Aeroset®, Abbott Laboratories, Chicago, IL, USA).
Measurement of the total antioxidant capacity
TAC of supernatant fractions was determined by using a novel automated measurement method developed by Erel.Citation5 Hydroxyl radicals, the most potent biological radicals, are produced in this method. In the assay, ferrous ion solution present in Reagent 1 is mixed with hydrogen peroxide, which is present in Reagent 2. The subsequently produced radicals such as brown-colored dianisidinyl radical cations produced by the hydroxyl radicals are also potent radicals. Using this method, the antioxidative effect of the sample is measured against the potent-free radical reactions initiated by the produced hydroxyl radicals. The assay has excellent precision values lower than 3%. The results are expressed in nmol Trolox Equiv./mg protein.
Measurement of total oxidant status
TOS of supernatant fractions was determined by using a novel automated measurement method developed by Erel.Citation6 Oxidants present in the sample oxidize the ferrous ion–odianisidine complex to ferric ion. The oxidation reaction is enhanced by glycerol molecules, which are abundantly present in the reaction medium. The ferric ion makes a colored complex with xylenol orange in an acidic medium. The color intensity, which can be measured spectrophotometrically, is related to the total amount of oxidant molecules present in the sample. The assay is calibrated with hydrogen peroxide, and the results are expressed in terms of nmol H2O2 Equiv./mg protein.
Oxidative stress index
The percent ratio of the TOS level to the TAC level was accepted as OSI. OSI values were calculated according to the following formulaCitation7:
Determination of myeloperoxidase activity
The MPO (EC 1.11.1.7) activity was determined using a 4-aminoantipyrine/phenol solution as the substrate for MPO-mediated oxidation by H2O2, and changes in absorbance at 510 nm were recorded.Citation8 One unit of MPO activity was defined as that which degraded 1 mol H2O2/min at 25°C. The results were expressed as mU/g protein.
Determination of catalase activity
Intestinal CAT activity was determined by using Goth's colorimetric method in which supernatant was incubated in H2O2 substrate and the enzymatic reaction was stopped by the addition of ammonium molybdate. The intensity of the yellow complex formed by molybdate and H2O2 was measured at 405 nm.Citation9 The results were expressed as MU/L.
Determination of nitrite oxide activity
NO measurement is very difficult in biological specimens; therefore, tissue nitrite (NO2–) and nitrate (NO3–) were estimated as an index of NO production. Samples were initially deproteinized with Somogyie agent. Total nitrite (nitrite + nitrate) was measured after conversion of nitrate to nitrite by copperier cadmium granules by a spectrophotometer at 545 nm.Citation10 Results were expressed as nmol/g wet tissue.
Determination of protein carbonyl activity
The PC contents were determined spectrophotometrically (Cintra 10 E, Austria) based on reaction of carbonyl group with 2,4-dinitrophenylhydrazine to form 2,4-dinitrophenylhydrazone.Citation11 The results were given as nanomoles of carbonyl per milligram of protein.
Histopathologic evaluation
The kidney of each animal was also taken for histological evaluation. Samples of kidney were placed in formalin and embedded in wax according to standard protocols. They were subsequently sectioned at 5 μm and stained with hematoxylin and eosin. Magnification of × 20 was used. Samples were then graded histologically according to the severity of injury by using a predetermined scoring system. The histological parameters evaluated were tubular necrosis, interstitial edema, loss of brush border, and cast formation. A minimum of 10 fields for each kidney slide were examined and evaluated for severity of the changes. The predetermined scoring system by Solez et al. was used.Citation12 The scoring system used was 0, absent; 1, present; and 2, marked. Total histological score per kidney was calculated by the addition of all scores. Histological analysis was performed by a blinded expert.
Statistical analysis
For statistical analyses, nonparametric independent group comparisons were made. For multiple comparisons, the Kruskal–Wallis test was used, and for comparisons between groups, the Mann–Whitney test was used if any statistical significance was found. A p-value of <0.05 was considered statistically significant. Data were expressed as mean ± SD.
RESULTS
All animals survived the experimental protocol. Renal functions such as serum urea and creatinine concentrations of rats subjected to 45 min ischemia and 6 hr reperfusion showed a marked deterioration compared with sham-operated rats (p < 0.001 for serum urea and p < 0.01 for serum creatinine). After I/R procedure, serum urea, creatinine, and cystatin C levels were significantly higher in I/R group compared to control and sham groups (p < 0.01). On the other hand, the serum urea, creatinine, and cystatin C levels were similar in groups of control and sham (p > 0.05), but higher in I/R + GO group than control and sham groups and the difference was significant statistically (p < 0.05). Oral application of GO treatment reduced serum urea, creatinine, and cystatin C levels significantly (p < 0.01 for all). Renal functional parameters are shown in . TAC and CAT activity in renal tissue was higher in sham group than I/R group and these were statistically significant (p < 0.05 for TAC and 0.001 for CAT). In addition, TAC and CAT activity in renal tissue were significantly higher in the treatment group than in the IR group (p < 0.01 for TAC and 0.001 for CAT). There were no statistically significant differences between the treatment group and the sham group (p > 0.05). TOS, OSI, and MPO levels in renal tissue were significantly lower in the treatment group than in the I/R group (p < 0.05, p < 0.05, and p < 0.001, respectively). However, TOS, OSI, and MPO levels were significantly higher in the I/R group than those in the sham group (p < 0.001, p < 0.01, and p < 0.001, respectively). There were not statistically significant differences between the treatment group and the sham group regarding to TOS, OSI, and MPO levels (p > 0.05 for all). In addition, NO and PC activities in renal tissue, which are oxidant markers, were higher in the rats subjected to I/R process as compared to controls. Nevertheless, NO and PC were significantly higher in I/R group than treatment group (p < 0.01). The results were summarized in .
TABLE 1. Serum clinical parameters in control, sham, I/R, and I/R + GO rats
TABLE 2. Oxidative and antioxidative parameters of the tissues
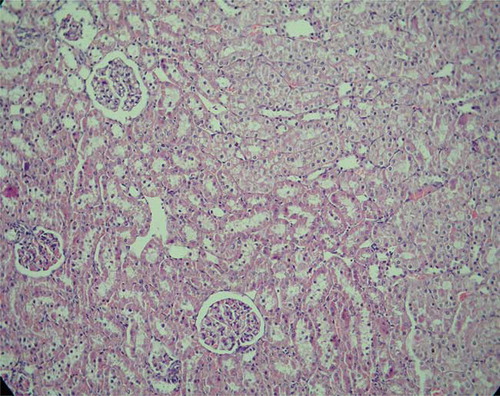
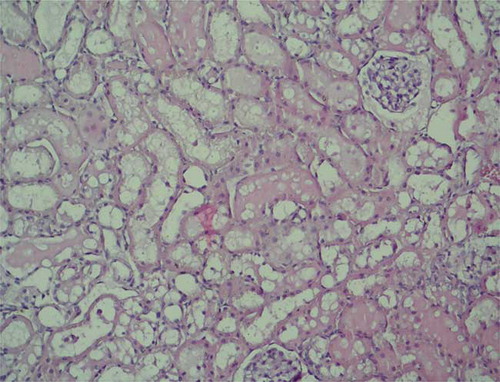
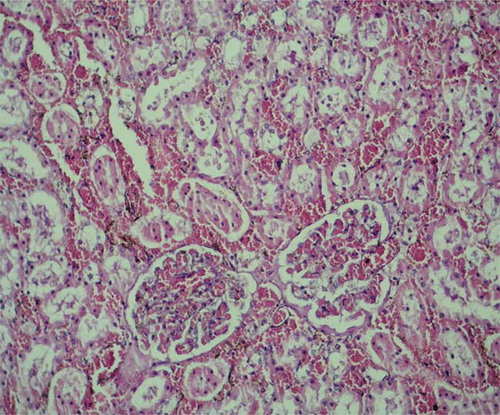
In histopathological evaluation, the renal tissues were observed normally with no pathological changes in the sham group (). Histopathological examination of the tissues in the control group revealed severe lesions such as tubular damage characterized by a loss of brush border, lumen dilatation or collapse, and cellular detachment from tubular basement membranes observed in the kidney of the rats (). In rats that received oral GO, these lesions were absent ().
DISCUSSION
Renal I/R is a major cause of acute renal failure and the underlying mechanisms of that are complex. Renal ischemia initiates an ordered series of events culminating in neutrophil adhesion to vascular endothelial cells and the entry of neutrophils into the extravascular space by the activation of neutrophils.Citation13 Activated neutrophils release oxygen-free radicals and release of free radicals has been shown to damage endothelial cells.Citation14 Also restoration of blood flow causes additional cellular injury by a significant local and systemic inflammatory injury by production of reactive oxygen radicals, although it is essential for the survival of ischemic renal tissue.Citation15 Therefore protection against I/R injury may be induced by administration of free radical scavengers and anti-inflammatory drugs.
In recent studies, it was shown that garlic or garlic extracts have effective antioxidant activity in different organs. Garlic, in the fresh or aged forms, has been used as a folk remedy for various disorders for thousands of years. Animal I/R studies suggest that garlic with its antioxidant and anti-inflammatory properties is a potential therapeutic agent to protect the function and structure of various organs against the injury from oxidative damage and neutrophil infiltration.Citation16
In our experimental study, renal ischemia for 45 min followed by reperfusion for 6 hr caused significant renal dysfunction as assessed by a marked increase in the serum concentrations of urea and creatinine in the control group. We also evaluated that oral GO treatment decreased the I/R-induced elevations of TOS, OSI, MPO, NO, and PC levels and it increased TAC and CAT levels in the renal tissue supernatants. Another antioxidant enzyme, CAT, was not significantly different among the groups, although it was the lowest in the rats subjected to I/R process. Increase in MPO activity in tissue is a good indicator of neutrophil infiltration.Citation17 Kabasakal et al. had shown that the increased MPO activity induced by I/R indicated that renal injury involves free radical formation.Citation18 In our experimental study we have shown a reduction in the activity of the enzyme MPO in the renal tissue of the rats in the oral GO-treated group compared with rats in the control group. In contrast, the activities of NO and PC were significantly higher in the rats given oral GO. Lipid peroxidation is implicated in the pathogenesis of post-ischemic tissue injuries.Citation18–22 Although NO has a function as a natural extra cellular scavenger of super oxide anions, it has a cytotoxic effect due to the inhibition of mitochondrial respiration.Citation23,Citation24 Moreover, cellular effects of reactive oxygen species are amplified during I/R by increased production of NO.Citation25 We have investigated the changes in the serum and tissue nitrite and nitrate levels, the final products of NO oxidation, and confirmed that serum NO levels were elevated after reperfusion. However, the rats undergoing I/R treatments with oral GO revealed significantly lower NO values compared to untreated rats. PC measurements are frequently used to assess the oxidative damage to proteins.Citation26 In this study, we measured the serum and tissue levels of PC, and PC level increased significantly after I/R process. Furthermore, the serum and tissue PC levels were significantly lower in the rats given oral GO than those without GO treatment. Oxidants and antioxidant capacity may be measured simultaneously to assess oxidative stress more exactly.
In a similar manner histological analyses showed less inflammatory infiltrate in kidneys of the rat in the treated group. Oxygen-free radical generation can be assessed by measuring the values of lipid peroxidation products. The measurement of TOS provides a sensitive index of lipid peroxidation and oxidative stress.Citation27 Our results confirm that renal I/R enhances oxidative stress, by either modulating production of free radicals and reactive oxygen species and, also, of toxic cytokines leading to inflammation and leukocyte infiltration or direct tissue damage, which are shown in previous studies.Citation28,Citation29 In this study, we also investigated the effects of oral GO on the parameters implicating oxidative and antioxidative status. We observed significantly decreased TOS and OSI levels and increased TAC levels with the administration of GO orally, which could be partly related to its antioxidant and free-radical scavenging effects.
We have revealed, for the first time in the pertinent literature, that kidney function test results are significantly better, the serum and tissue antioxidant enzymes levels are significantly high, and the tissue oxidant enzyme levels were significantly lower in the rats treated with oral GO prior to I/R process than the rats only subjected to the I/R process. The protective effects of oral administration of GO against I/R injury seems to be associated with its anti-inflammatory effects. As an inference, it seems possible to conclude from this study that oral administration of GO may have beneficial effects to prevent renal I/R injury, and garlic and garlic extracts may be used in human studies in the future.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Donnahoo KK, Meldrum DR, Shenkar R, Chung CS, Abraham E, Harken AH. Early renal ischemia, with or without reperfusion, activates NFkappaB and increases TNF-alpha bioactivity in the kidney. J Urol. 2000;163:1328–1332.
- Sivarajah A, Chatterjee PK, Patel NS, Agonists of peroxisome-proliferator activated receptor-gamma reduce renal ischemia/reperfusion injury. Am J Nephrol. 2003;23:267–276.
- Parks DA, Granger DN. Contributions of ischemia and reperfusion to mucosal lesion formation. Am J Physiol. 1986;250:G749–G753.
- Bhagyalakshmi N, Thimmaraju R, Venkatachalam L, Murthy KN, Sreedhar RV. Nutraceutical applications of garlic and the intervention of biotechnology. Crit Rev Food Sci Nutr. 2005;45:607–621.
- Erel O. A novel automated method to measure total antioxidant response against potent free radical reactions. Clin Biochem. 2004;37:112–119.
- Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005;38:1103–1111.
- Bolukbas C, Bolukbas FF, Horoz M, Aslan M, Celik H, Erel O. Increased oxidative stress associated with the severity of the liver disease in various forms of hepatitis B virus infection. BMC Infect Dis. 2005;5:95.
- Wei H, Frenkel K. Relationship of oxidative events and DNA oxidation in SENCAR mice to in vivo promoting activity of phorbol ester-type tumor promoters. Carcinogenesis. 1993;14:1195–1201.
- Goth L. A simple method for determination of serum catalase activity and revision of reference range. Clin Chim Acta. 1991;196:143–151.
- Cortas NK, Wakid NW. Determination of inorganic nitrate in serum and urine by a kinetic cadmium-reduction method. Clin Chem. 1990;36:1440–1443.
- Levine RL, Garland D, Oliver CN, Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990;186:464–478.
- Solez K, Morel-Maroger L, Sraer JD. The morphology of “acute tubular necrosis” in man: Analysis of 57 renal biopsies and a comparison with the glycerol model. Medicine. 1979;58:362–376.
- Lien YH, Yong KC, Cho C, Igarashi S, Lai LW. S1P(1)-selective agonist, SEW2871, ameliorates ischemic acute renal failure. Kidney Int. 2006;69:1601–1608.
- Basivireddy J, Jacob M, Pulimood AB, Balasubramanian KA. Indomethacin-induced renal damage: Role of oxygen free radicals. Biochem Pharmacol. 2004;67:587–599.
- Haase E, Bigam DL, Nakonechny QB, Jewell LD, Korbutt G, Cheung PY. Resuscitation with 100% oxygen causes intestinal glutathione oxidation and reoxygenation injury in asphyxiated newborn piglets. Ann Surg. 2004;240:364–373.
- Sener G, Sakarcan A, Yegen BC. Role of garlic in the prevention of ischemia-reperfusion injury. Mol Nutr Food Res. 2007;51:1345–1352.
- Faith M, Sukumaran A, Pulimood AB, Jacob M. How reliable an indicator of inflammation is myeloperoxidase activity? Clin Chim Acta. 2008;396:23–25.
- Kabasakal L, Sehirli O, Cetinel S, Cikler E, Gedik N, Sener G. Protective effect of aqueous garlic extract against renal ischemia/reperfusion injury in rats. J Med Food. 2005;8:319–326.
- Eschwege P, Paradis V, Conti M, In situ detection of lipid peroxidation by-products as markers of renal ischemia injuries in rat kidneys. J Urol. 1999;162:553–557.
- Paller MS, Hoidal JR, Ferris TF. Oxygen free radicals in ischemic acute renal failure in the rat. J Clin Invest. 1984;74:1156–1164.
- Green CJ, Healing G, Simpkin S, Lunec J, Fuller BJ. Increased susceptibility to lipid peroxidation in rabbit kidneys: A consequence of warm ischemia and subsequent reperfusion. Comp Biochem Physiol. 1986;83:603–606.
- Cristol JP, Thiemermann C, Guearin MC, Torreills J, de Paulet AC. L-Arginine infusion after ischemia-reperfusion of rat kidney enhances lipid peroxidation. J Lipid Mediat Cell Signal. 1996;13:9–17.
- Chatterjee PK, Patel NS, Kvale EO, Inhibition of inducible nitric oxide synthase reduces renal ischemia/reperfusion injury. Kidney Int. 2002;61:862–871.
- Isobe M, Katsuramaki T, Hirata K, Kimura H, Nagayama M, Matsuno T. Beneficial effects of inducible nitric oxide synthase inhibitor on reperfusion injury in the pig liver. Transplantation. 1999;68:803–813.
- Noiri E, Nakao A, Uchida K, Oxidative and nitrosative stress in acute renal ischemia. Am J Physiol Renal Physiol. 2001;281:948–957.
- Berlett BS, Stadtman ER. Protein oxidation in aging, disease, and oxidative stress. J Biol Chem. 1997;272:20313–20316.
- Aycicek A, Ipek A. Maternal active or passive smoking causes oxidative stress in cord blood. Eur J Pediatr. 2008;167:81–85.
- Uz E, Karatas OF, Mete E, The effect of dietary ginger (Zingiber officinals Rosc) on renal ischemia/reperfusion injury in rat kidneys. Ren Fail. 2009;31:251–260.
- Senturk H, Kabay S, Bayramoglu G, Silymarin attenuates the renal ischemia/reperfusion injury-induced morphological changes in the rat kidney. World J Urol. 2008;26:401–407.