Abstract
Aim: Nephrotoxicity is a major side effect of cisplatin (Cis), a widely used chemotherapeutic drug. Recent studies have strongly suggested that inflammatory mechanisms may play an important role in the pathogenesis of Cis nephrotoxicity. Rosiglitazone (Ros), a peroxisome proliferator-activated receptor-gamma agonist has been recently demonstrated to regulate inflammation by modulating the production of inflammatory mediators and adhesion molecules. The aim of this study was to evaluate the effect of Ros on the prevention of Cis-induced nephrotoxicity. Methods: Eighteen male Sprague–Dawley rats weighing 150–200 g were included in the study. The rats were randomly divided into three groups: group 1: Cis-treated group; group 2: Cis–Ros-treated group; group 3: saline-treated group. Blood urea nitrogen (BUN) and serum creatinine concentrations were measured. In addition, extent of histological renal tubular injury in each animal was graded histologically. Results: Mean BUN and serum creatinine concentrations were significantly lower in group 3 than in group 1 (p < 0.05) and group 2 (p < 0.05). There were no significant differences in terms of BUN and serum creatinine concentrations between groups 1 and 2 (p > 0.05). Acute tubular injury with karyomegalic changes in corticomedullary junction was significantly higher in groups 1 and 2 than group 3 (p < 0.05). However, there were no significant differences between groups 1 and 2 (p > 0.05). Conclusion: This study indicates that post-insult administration of Ros does not seem to have a beneficial effect on prevention and severity of nephrotoxicity induced by Cis.
INTRODUCTION
Cisplatin (Cis) is a platinum chemotherapeutic used in a variety of malignancies; however, severe nephrotoxicity after Cis treatment is the dose-limiting adverse reaction. Although several mechanisms have been proposed for Cis-induced nephrotoxicity, including mitochondrial dysfunction, direct DNA damage, activation of caspase formation of reactive oxygen species, effects on the endoplasmic reticulum increased lipid peroxidation activation, and the mechanisms underlying Cis-mediated nephrotoxicity are not fully understood.Citation1–5 In addition, recent studies suggested the role of tumor necrosis factor (TNF)-α-mediated apoptotic pathways.Citation6–9
Peroxisome proliferator-activated receptor-gamma (PPAR-gamma) is a member of PPARs, which belong to nuclear receptor superfamily.Citation10 Upon activation by selective ligands, PPAR-gamma interacts with retinoid X receptor to form heterodimers, which bind PPAR response elements (PPREs) to activate or suppress target genes.Citation11 Rosiglitazone (Ros) belongs to thiazolidinediones new class of antidiabetic drugs, which are PPAR-gamma agonists. Previous studies have shown that agonists of the PPAR play an important role in the regulation of inflammatory responses.Citation12,Citation13 These ligands inhibit inflammation by regulating the activities of transcription factor activator protein-1 or nuclear factor-κB (NF-κB).Citation10,Citation11 Because of the proposed role of cytokines in Cis-induced renal injury, we aimed to examine whether PPAR-gamma agonist, Ros, could ameliorate or prevent Cis-induced nephrotoxicity.
MATERIALS AND METHODS
Animals and experimental design
A total of 18 adult male Wistar rats (weighing 150–200 g) were used in the study. They were housed separately in metal cages. The animals were kept under the experimental housing and normal feeding conditions for 5 days prior to the test. The animal room was maintained at 22 ± 2°C, 60 ± 5% relative humidity, and 12:12 hr light–dark cycle. Food and water were available ad libitum. The rats were randomly divided into three groups: group 1 (n = 6) was given single Cis dose (7.5 mg/kg) intraperitoneally that was previously dissolved in saline solution (1.5 mg/mL); group 2 (n = 6) 7.5 mg/kg/intraperitoneally (i.p). Cis plus 2.5 mg/kg Ros through an oral gavage (administered 1 hr before Cis and once daily over a 7-day period); and group 3 (n = 6) 1 mL saline. After the treatment, the animals were treated with intramuscular 60 mg/kg ketamine (Alfamine 10%, Ege-Vet, İzmir, Turkey) for anesthesia, and heart blood was collected to evaluate the renal functions. Seven days after the last Cis injection, the rats were killed. The experimental protocol was approved by the Ondokuz Mayis University Local Ethical Committee for Animal Studies.
Histological examination of the kidney
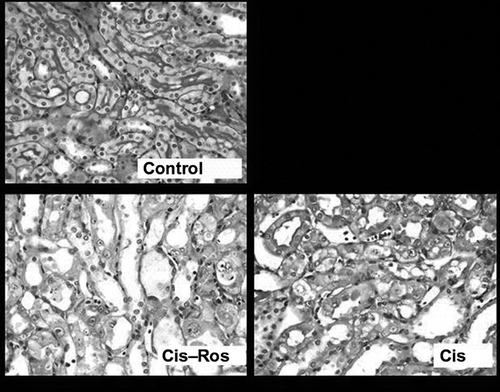
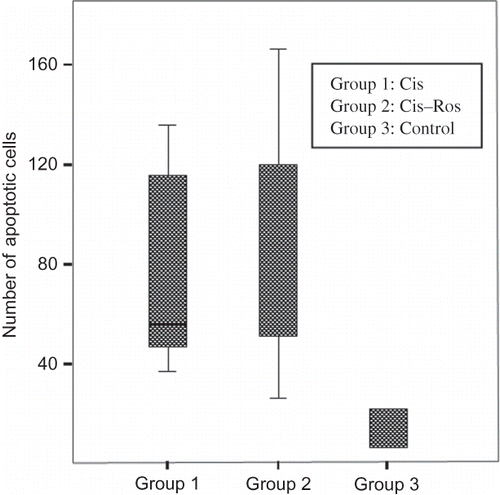
One kidney was removed from each rat for histological examination. The kidney was incised coronal axis with a razor blade and transferred immediately into 4% neutral formalin. After fixation for 24 hr, the tissues were embedded in paraffin and then sectioned 4 μm. The sections were stained with periodic acid-Schiff (PAS) and examined using light microscopy (Olympus BX50 microscope, Olympus Optical Company, Tokyo, Japan), as described previously.Citation6,Citation14 Since the morphological abnormalities in tubules are mainly localized in the corticomedullary junction, and the outer cortex tubules and glomeruli of the kidney do not exhibit major histological alterations, only the inner cortex and outer medullar regions were examined in detail. Tubular necrosis profile was assigned when there were the following findings: flattening of tubular epithelium, loss of brush border, apical blebbing, and desquamation of individual tubular epithelial cells, dilation of tubular lumen, and the presence of intratubular debris or calcification. The bizarre karyomegalic histopathology in tubules was also evaluated. Tubular necrosis and karyomegaly were assessed semiquantitatively in whole corticomedullary junction zone with 20× magnification objective. Tubular injury was scored on a scale of 0–4 based on the percentage of tubules affected (ranging from 0, normal; 1, <10%; 2, 10–25%; 3, 26–75%; 4, >75%) and an average was determined for each section. Apoptotic bodies as a quantitative parameter was counted in 10 consecutive but non-overlapping high-power fields (with 40× magnification objective) and the total number of apoptotic bodies was calculated for each section. Apoptotic bodies were assessed morphologically as defined strictly by chromatin condensation or nuclear fragmentation with eosinophilic globules enclosed in a clear space. The evaluation started from one point chosen arbitrarily in the corticomedullary junction. From this point, the stage was moved in only one direction along this row and the consecutive fields were read. Care was taken not to score the same area or to count the same apoptotic profile twice. Without knowledge of the experimental group, all evaluations were made on coded sections by a pathologist.
Statistical methods
Data are given as median (range). To compare groups Kruskal–Wallis analysis of variance and Mann–Whitney U-test were used where appropriate. p-Values less than 0.05 were considered significant. Data were analyzed using SPSS 15.0 software.
RESULTS
Median BUN and serum creatinine concentrations in Cis and Cis–Ros groups were significantly higher than control group but there was no significant difference between Cis and Cis–Ros groups (). Histopathologic examination revealed prominent acute tubular injury with karyomegalic changes in corticomedullary junction in Cis and Cis–Ros groups when compared with the saline group (p = 0.04) (). For acute tubular necrosis, semiquantitative scores of Cis and Cis–Ros groups were 3.2 (range: 2.8–3.4) and 3.5 (range: 3–3.9), respectively. For karyomegaly, semiquantitative scores of Cis and Cis–Ros groups were 2.7 (range: 1.8–2.8) and 2.8 (range: 1–3.6), respectively. Although the group pretreated with Ros seemed to have more tubular injury, the difference between these groups in terms of tubular injury was statistically insignificant. Renal tubular epithelial cell apoptosis induced by Cis was quantified also. Contrast the saline group, increased apoptosis in tubular epithelium was apparent after injection of Cis (p = 0.004). However, there was no significant difference in the number of apoptotic cells between Cis and Cis–Ros group (p > 0.05) ().
TABLE 1. Biochemical and histopathologic findings of the treatment groups; data are presented as median (range)
DISCUSSION
Cellular and molecular mechanisms responsible for Cis-induced nephrotoxicity are not well understood but several mechanisms involving with oxidative stress, DNA damage, and apoptosis have been proposed.Citation1–5 Recent studies indicated the role of inflammation and expression of cytokines and chemokines in the pathogenesis of Cis-induced nephrotoxicity.Citation6–9 It has been shown that systemic injection of Cis administration was associated with increased expression for mRNA as well as the protein content of TNF-α and other pro-inflammatory cytokines such as TGF-b, RANTES, MCP-1, and IL-1 in renal tissues of mice.Citation7 There are also reports indicating that Cis treatment induces significant activation of NF-κB and remarkable increases in TNF-α production in kidneys, and the inhibition of NF-κB activation and TNF-α production attenuated the Cis-induced renal injury.Citation7,Citation8,Citation15 Along with the effects on adipocyte differentiation and glucose homeostasis, PPAR-gamma agonists such as Ros have recently been shown to regulate inflammatory responses. Previous studies have shown that PPAR-gamma agonists inhibit the expression of TNF-α and other pro-inflammatory cytokines, chemokines, and adhesion molecules in macrophages and other cell types.Citation10,Citation11,Citation16,Citation17 These effects result from the targeting of multiple pathways and include inhibition of NF-κB-dependent responses.Citation18–20 Various studies have shown that PPAR-gamma agonists are efficacious in slowing the progression of glomerulosclerosis (diabetic and nondiabetic) and ischemia–reperfusion injury.Citation21–23
Due to its anti-inflammatory activity, Ros may have beneficial effects on the prevention and treatment of Cis-induced nephrotoxicity. However, the data about the role of Ros on the Cis-induced nephrotoxicity are limited.Citation24 A recent study showed that pretreatment with Ros significantly decreased the damage to renal function and histological pathology through the suppression of TNF-α overproduction and NF-κB activation after Cis administration. Contrary to their findings, the results of this study demonstrated that Ros treatment of rat did not improve Cis-induced renal dysfunction and organ damage as confirmed by biochemical assays and histopathologic examination. The discrepancy between these two studies may due to the dosage of Ros administered. In the study by Lee et al.,Citation24 Ros was administered at a dose of 10 mg/kg once a day for 3 days to the mice. In our study rats received the Ros at a dose of 2.5 mg/kg (first dose before Cis administration and over 7 days).
In conclusion, post-insult administration of Ros does not seem to have a beneficial effect on prevention and severity of nephrotoxicity induced by Cis. Further studies evaluating the effect of different Ros dosages in Cis-induced nephrotoxicity are needed.
Acknowledgments
This study was supported by Ondokuz Mayis University Research Fund (Samsun, Turkey; Grant No: 60-T.406).
REFERENCES
- Sugiyama S, Hayakawa M, Kato T, Hanaki Y, Shimizu K, Ozawa T. Adverse effects of anti-tumor drug, cisplatin on rat kidney mitochondria: Disturbances in glutathione peroxidase activity. Biochem Biophys Res Commun. 1989;159:1121–1127.
- Leibbrandt ME, Wolfgang GH, Metz AL, Ozobia AA, Haskins JR. Critical subcellular targets of cisplatin and related platinum analogs in rat renal proximal tubule cells. Kidney Int. 1995;48:761–770.
- Kaushal GP, Kaushal V, Hong X, Shah SV. Role and regulation of activation of caspases in cisplatin induced injury to renal tubular epithelial cells. Kidney Int. 2001;60:1726–1736.
- Matsushima H, Yonemura K, Ohishi K, Hishida A. The role of oxygen free radicals in cisplatin induced acute renal failure in rats. J Lab Clin Med. 1998;131:518–526.
- Liu H, Baliga R. Endoplasmic reticulum stress-associated caspase 12 mediates cisplatin-induced LLC-PK1 cell apoptosis. J Am Soc Nephrol. 2005;16:1985–1992.
- Moraes LA, Piqueras L, Bishop-Bailey D. Peroxisome proliferator activated receptors and inflammation. Pharmacol Ther. 2006;110:371–385.
- Willson TM, Lambert MH, Kliewer SA. Peroxisome proliferator-activated receptor gamma and metabolic disease. Annu Rev Biochem. 2001;70:341–367.
- Welch JS, Ricote M, Akiyama TE, Gonzalez FJ, Glass CK. PPARgamma and PPARdelta negatively regulate specific subsets of lipopolysaccharide and IFN-gamma target genes in macrophages. Proc Natl Acad Sci USA. 2003;100:6712–6717.
- Smeets PJ, Planavila A, van der Vusse GJ, van Bilsen M. Peroxisome proliferator-activated receptors and inflammation: Take it to heart. Acta Physiol (Oxf). 2007;191:171–188.
- Jiang C, Ting AT, Seed B. PPAR-γ agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391:82–86.
- Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-γ is a negative regulator of macrophage activation. Nature. 1998;391:79–82.
- Kopple JD, Ding H, Letoha A, L-carnitine ameliorates gentamicin-induced renal injury in rats. Nephrol Dial Transplant. 2002;17:2122–2131.
- Ramesh G, Reeves WB. TNFR2-mediated apoptosis and necrosis in cisplatin-induced acute renal failure. Am J Physiol Renal Physiol. 2003;285:F610–F618.
- Zahng B, Ramesh G, Norbury CC, Reeves WB. Cisplatin-induced nephrotoxicity is mediated by tumor necrosis factor-alpha produced by renal parenchymal cells. Kidney Int. 2007;72:5–7.
- Mishima K, Baba A, Matsuo M, Protective effect of cyclic AMP against cisplatin-induced nephrotoxicity. Free Radic Biol Med. 2006;40:1564–1577.
- Marx N, Mach F, Sauty A, Peroxisome proliferator-activated receptor-gamma activators inhibit IFN-gamma-induced expression of the T cell-active CXC chemokines IP-10, Mig, and I-TAC in human endothelial cells. J Immunol. 2000;164:6503–6508.
- Pasceri V, Wu HD, Willerson JT, Yeh ET. Modulation of vascular inflammation in vitro and in vivo by peroxisome proliferator-activated receptor-gamma activators. Circulation. 2000;101:235–238.
- Eligini S, Banfi C, Brambilla M, 15-Deoxy-delta 12,14-prostaglandin J2 inhibits tissue factor expression in human macrophages and endothelial cells: Evidence for ERK1/2 signaling pathway blockade. Thromb Haemost. 2002;88:524–532.
- Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: Integrating mammalian biology. Cell. 2001;104:487–501.
- van de Stolpe A, van der Saag PT. Intercellular adhesion molecule-1. J Mol Med. 1996;74:13–33.
- Qian Y, Li S, Ye S, Renoprotective effect of rosiglitazone through the suppression of renal intercellular adhesion molecule-1 expression in streptozotocin-induced diabetic rats. J Endocrinol Invest. 2008;12:1069–1074.
- Ma LJ, Marcantoni C, Linton MF, Fazio S, Fogo AB. Peroxisome proliferator-activated receptor-gamma agonist troglitazone protects against nondiabetic glomerulosclerosis in rats. Kidney Int. 2001;59:1899–1910.
- Sivarajah A, Chatterjee PK, Patel NS, Agonists of peroxisome proliferator activated receptor-gamma reduce renal ischemia/reperfusion injury. Am J Nephrol. 2003;23:267–276.
- Lee S, Kim W, Moon SO, Rosiglitazone ameliorates cisplatin-induced renal injury in mice. Nephrol Dial Transplant. 2006;21:2096–2105.