Abstract
Background: Podocyte loss plays an important role in the pathogenesis of diabetic nephropathy, but counting the number of glomerular podocyte in renal biopsy specimen is a labor-intensive task. We study whether intra-renal and urinary messenger RNA expression of podocyte-associated molecules could be used to estimate glomerular podocyte number in patients with diabetic nephropathy. Method: We studied 21 consecutive patients with biopsy-proven diabetic nephropathy. The intra-renal and urinary mRNA expression of nephrin, podocin, and synaptopodin were measured by real-time quantitative polymerase chain reaction. Podocyte number was determined in micro-dissected glomerulus. The degree of histological scarring was quantified by morphometric analysis. Results: Glomerular podocyte number correlated with intra-renal expression of nephrin (r = 0.510, p = 0.044), podocin (r = 0.605, p = 0.013), and synaptopodin (r = 0.480, p = 0.060). Glomerular podocyte number also significantly correlated with urinary expression of synaptopodin (r = 0.595, p = 0.019) but not other targets. Baseline renal function correlated with intra-renal expression of nephrin (r = 0.617, p = 0.005), synaptopodin (r = 0.474, p = 0.040), and podocin (r = 0.443, p = 0.057). The degree of tubulointerstitial scarring also inversely correlated with intra-renal expression of nephrin (r = −0.462, p = 0.047), podocin (r = −0.458, p = 0.049), and synaptopodin (r = −0.500, p = 0.029) but not with urinary gene expression. Conclusion: Intra-renal expression of podocyte-associated molecules correlated with glomerular podocyte number, renal function, and tubulointerstitial scarring. The results suggest that intra-renal, but not urinary expression of podocyte-associated molecules, might be used to assess the degree of podocyte loss in diabetic nephropathy.
INTRODUCTION
Diabetic nephropathy is one of the leading causes of end-stage renal disease in western world and has a trend to spread in developing countries.Citation1,Citation2 The pathogenesis of diabetic nephropathy is not fully elucidated. Studies in recent years showed that podocyte plays an important role in the progression of diabetic nephropathy.Citation3 A number of studies have found an inverse correlation between the number of podocytes in glomeruli and the degree of proteinuria,Citation4–6 and a decrease in podocyte number per glomerulus is related to glomerulosclerosis and contributes to the progression of diabetic nephropathy.Citation7–10 Besides apoptosis, detachment into urine seems to be an important route of podocyte loss. In fact, viable podocytes could be detected in the urine in many kidney diseases.Citation11–14
Measurement of podocyte loss in patients with diabetic nephropathy has long been hampered by the need of renal biopsies and counting of podocyte number by immunohistochemistry followed with labor-intensive morphometric technique. Quantification of intra-renal expression of genes specifically found in podocyte – for example, nephrin – is a logical alternative, but the technique has not been validated. Urinary detection of podocyte injury has also been implicated recently.Citation15 Efforts on detection of podocytes and podocyte-associated molecules in the urine by immunofluorescence and western blotting methods,Citation16–18 however, are not practical in clinical use. On this aspect, our previous study found increased mRNA expression levels of podocyte-associated molecules in the urine sediment of patients with diabetic nephropathy, and the degree of the expression are correlated with several clinical parameters.Citation19,Citation20 In this study, we examined whether urinary and glomerular mRNA expression of podocyte-associated molecules correlates with the degree of podocyte loss in patients with diabetic nephropathy.
PATIENTS AND METHODS
Subjects
We studied 21 consecutive diabetic patients who had renal biopsy in our hospital. The urinary expression of podocyte-associated molecules of 15 patients has been reported in our previous study.Citation19 All patients had biopsy-proved diabetic glomerulosclerosis and 16 of them were in moderate to advanced stage.Citation21 A whole-stream early morning urine specimen was collected on biopsy day for gene expression study. Clinical data, including serum creatinine and 24-hour urine protein, were recorded. The glomerular filtration rate (GFR) was estimated by a standard equation.Citation22 This study adheres to the Declaration of Helsinki and informed consents have been obtained from all subjects.
Estimation of average podocyte number per glomerulus
Shortly after kidney biopsy, part of the tissue was embedded by Optimal Cutting Temperature (OCT) compound (Sakura, Torrance, CA, USA) and kept at −80°C under RNase-free condition for intra-renal gene expression. The tissue was also fixed in 10% neutral buffered formaldehyde overnight and then dehydrated by alcohol and embedded in paraffin.
We used the Weibel and GomezCitation23 method to estimate average podocyte number per glomerulus by point-counting technique. Podocyte nucleuses were recognized by WT-1 immunostaining and we assumed all podocyte nucleuses were positive for WT-1 in this study.Citation24 For WT-1 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) immunostaining, 4-μm thick paraffin sections were stained using ImmunoCruz™ Staining Systems (Santa Cruz Biotechnology) according to manufacturer's instruction. We calculated the average podocyte number per glomerulus by multiplying the mean numerical density of podocyte nucleus (NV) and the mean glomerular volume (VG) together. NV was estimated by the following formulaCitation23:
where NA is the numerical density of nuclear profiles per glomerular area, and can be calculated by point counting: NA = N/[PC × (D/M)2], where N is nuclei number, PC is coarse point in glomerular profile, D is the distance between coarse points, and M is magnification. VV is the volume fraction of podocyte nuclei per glomerulus and can be calculated by VV = PF/(PC × 16), where PF is fine points hitting nuclei and 16 is ratio of fine points to coarse points. K is a size distribution coefficient and close to 1 and can be neglected, and β is a shape constant and in this analysis, 1.55. VG was estimated by a method introduced previously, which used two arbitrary parallel sections.Citation25
Morphometric study of kidney biopsy
Jones' silver staining was performed on 5-μm thick sections of renal biopsy specimen of each patient. As previously described,Citation26 we used computerized image analysis method to semi-quantify nephrosclerosis. Briefly, a Leica Twin Pro image analysis system (Leica Microsystems, Wetzlar, Germany) was connected to a Leica DC500 digital camera on a Leica DMRXA2 microscope working with a 40× objective (final calibration: 0.258 mm/pixel) and to a microcomputer for storage of the morphometric measurements and to perform image analysis by using image-analyzing software (MetaMorph 4.0; Universal Imaging Corporation™, Downingtown, PA, USA). Ten glomeruli and 10 randomly selected tubular interstitial areas were assessed in each patient and the average percentage of scarred glomerular and tubulointerstitial areas, as represented by the percentage of the area with positive staining, were computed for each patient.
Laser micro-dissection
We further performed laser micro-dissection to determine the glomerular mRNA expression of podocyte-associated molecules. Briefly, 8- to –10-μm thick cryosections were prepared on a cryostat (Leica Microsystems) using disposable microtome blades (Leica) under RNase-free conditions. Sections were then mounted on glass slides covered with a polyethylene naphtholate membrane (PALM Microlaser Technologies, Bernried, Germany) and were treated with UV irradiation and RNase Away (Invitrogen, Life Technologies, Carlsbad, CA, USA) prior to use. After being fixed in 70% ethanol for 2 minutes, the sections were stained according to the following protocol: DEPC-water 1 minute, Mayer's hematoxylin (Sigma Chemical Company, St. Louis, MO, USA) solution 30 seconds, RNase-free water 1 minute, 50% ethanol 1 minute, 70% ethanol 1 minute, 100% ethanol 1-minute twice, and air dry 10 minutes.
Next, PALM® Microlaser Systems (PALM, Wolfratshausen, Germany), which is equipped with a pulsed high-quality laser beam, computer-controlled microscope stage, and micro-manipulator, was used to perform micro-dissection of prepared slides. Under direct visual control, 20 ± 5 glomerular areas and proportional randomly selected tubulointerstitial area of each case were isolated by the focused nitrogen laser beam and then catapulted into different micro-centrifuge caps, which were filled with guanidine thiocyanate-containing lysis buffer (buffer RLT, Qiagen Inc., Ontario, Canada). The tissue lysate of isolated glomerulus and tubulointerstitium were then stored at −80°C until RNA extraction was performed.
Measurement of urinary and intra-renal gene expression
Urinary messenger RNA extraction was performed according to the method described previously.Citation27 In brief, urine specimens were centrifuged at 3000 g for 30 minutes and at 13,000 g for 5 minutes at 4°C shortly after collection. Supernatant was then discarded and the urinary cell pellets were lysed by RNA lysis buffer (Qiagen Inc.). Specimens were stored in −80°C until use. RNeasy Mini Kit (Qiagen Inc.) and RNeasy Micro Kit were used to extract total RNA from urinary sediment and micro-dissected tissue, respectively, according to manufacturer's protocol. DNase was used to wipe-off probable genomic DNA. We confirmed the purity of urinary RNA by the relative absorbance at 260/280 nm ratio using a spectrometer. Our previous data have shown the integrity of RNA isolated from urinary sediment by this method is adequate for RT-QPCR.Citation28
For reverse transcription, 5 μL total RNA was mixed with 1-μL random primers (150 ng), 1-μL dNTP mix (10 mM each), 4-μL 5 × first-strand buffer, 2 μL DTT (0.1 M), 1-μL Superscript II RNase H Reverse Transcriptase (all from Intrivogen™, Life Technologies, Philadelphia, PA), and make up to 20 μL with H2O. Reverse transcription was performed at 65°C for 5 minutes, 25°C for 10 minutes, 42°C for 50 minutes, and then inactivate reaction at 70°C for 10 minutes. The resulting cDNA was stored in −80°C until use.
We quantified urinary and intra-renal expression of nephrin, podocin, and synaptopodin using the ABI Prism 7700 Sequence Detection System (Applied Biosystems, Foster City, CA, USA). Commercially available Taqman primers and probes, including two unlabeled PCR primers and one FAM™ dye-labeled TaqMan® MGB probe were used for all the target genes (all from Applied Biosystems). The primer and probe set was deliberately designed across the intron–exon boundary so as not to detect probable genomic DNA. For RT-QPCR, 10 μL universal master mix, 1-μL primer and probe set, 2 μL cDNA, and 7 μL H2O were mixed to make a 20-μL reaction volume. Each sample was run in triplicate. RT-QPCR were performed at 50°C for 2 minutes, 95°C for 10 minutes, followed by 40 cycles at 95°C for 15 seconds, and 60°C for 1 minute. 18S rRNA (Applied Biosystems) was used as housekeeping gene to normalize the mRNA expression level of each target gene. Results were analyzed with Sequence Detection Software version 1.7 (Applied Biosystems). In order to calculate the differences of expression level for each target genes among samples, the ΔΔCT method for relative quantitation is used according to manufacturer's manual.Citation29 For the amplification efficiency of all TaqMan Gene Expression Assays is equivalent to any other target assay, it is not necessary to validate the PCR efficiencies of the target and endogenous control.Citation30
Clinical follow-up
After kidney biopsy, all patients were followed for at least 12 months. Clinical management was decided by individual nephrologist and not affected by the study. Serum creatinine, estimated GFR, and proteinuria were assessed at least every 4 months. The rate of GFR decline was calculated by the least square regression method.
Statistical analysis
Statistical analysis was performed by SPSS for Windows software version 11.0 (SPSS Inc., Chicago, IL, USA). All the results were presented in mean ± SD for data normally distributed and median (lower and upper quartiles) for the others. Since data of gene expression levels were highly skewed, log transformation and Pearson's correlation were also used. A p-value of below 0.05 was considered statistically significant. All probabilities were two-tailed.
RESULTS
The demographic and baseline clinical data of the study subjects are summarized in . Morphometric study showed that the percentage of the scarred glomerular and tubulointerstitial areas were 27.5 ± 16.1% and 14.5 ± 6.1%, respectively. Podocyte number per glomerulus of the study subjects was 477.5 ± 258.8.
TABLE 1. Demographic and baseline clinical data
Relation between podocyte number and gene expression
The correlations between glomerular podocyte number and intra-renal mRNA expression of podocyte-associated molecules are summarized in (a). In short, podocyte number significantly correlated with intra-renal expression of nephrin (r = 0.510, p = 0.044) and podocin (r = 0.605, p = 0.013). There was also a trend of correlation between podocyte number and intra-renal synaptopodin expression (r = 0.480, p = 0.060), although the result did not reach statistical significance.
FIGURE 1. Relations between glomerular podocyte number and (a) intra-renal mRNA expression and (b) urinary mRNA expression of podocyte-associated molecules (nephrin, podocin, and synaptopodin). Gene expression data are depicted as the ratio to control.
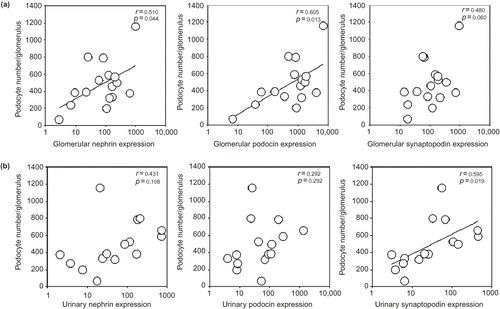
The correlations between glomerular podocyte number and urinary mRNA expression of podocyte-associated molecules are summarized in (b). There was a significant correlation between glomerular podocyte number and urinary expression of synaptopodin (r = 0.595, p = 0.019), but not nephrin (r = 0.431, p = 0.108) or podocin (r = 0.292, p = 0.292). There was no significant correlation between intra-renal and urinary expression of any of the gene studied ().
Relation with baseline clinical and histological parameters
There were no significant correlations between glomerular podocyte number and baseline-estimated GFR and proteinuria (details not shown). However, baseline-estimated GFR correlated with intra-renal expression of nephrin (r = 0.617, p = 0.005), synaptopodin (r = 0.474, p = 0.040), and podocin (r = 0.443, p = 0.057; ), although the last correlation did not reach statistical significance. On the contrary, no significant correlation was found between proteinuria and intra-renal expression of any of the target genes (details not shown).
FIGURE 3. Relations between and intra-renal mRNA expression of podocyte-associated molecules (nephrin, podocin, and synaptopodin) and (a) baseline glomerular filtration rate (GFR); and (b) degree of tubulointerstitial scarring in renal biopsy. Gene expression data are depicted as the ratio to control.
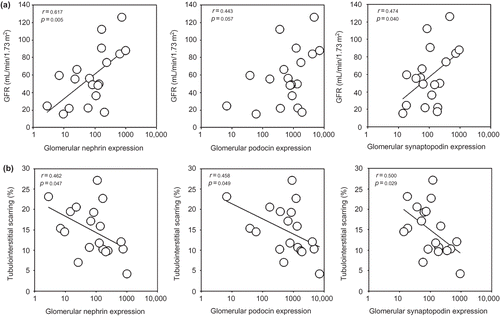
Baseline-estimated GFR inversely correlated with urinary expression of podocin (r = −0.497, p = 0.036), but not nephrin (r = −0.295, p = 0.234) or synaptopodin (r = −0.272, p = 0.274). Proteinuria correlated with urinary expression of nephrin (r = 0.532, p = 0.023), but not synaptopodin (r = 0.449, p = 0.061) or podocin (r = 0.286, p = 0.251).
Glomerular podocyte number inversely correlated with tubulointerstitial scarring (r = −0.649, p = 0.004) and glomerular scarring (r = −0.465, p = 0.052), although the latter did not reach statistical significance. There were also significant correlations between the degree of tubulointerstitial scarring and intra-renal expression of nephrin (r = −0.462, p = 0.047), podocin (r = −0.458, p = 0.049), and synaptopodin (r = 0.500, p = 0.029; ). In contrast, the degree of glomerular scarring did not significantly correlate with intra-renal gene expression of podocyte-associated molecules. Renal scarring did not correlate with urinary expression of any of the gene studied (details not shown).
Relation with GFR decline rate
The average duration of follow-up was 27.8 ± 11.5 months, and the average GFR decline rate was −0.98 ± 1.23 mL/min per 1.73 m2/month. The rate of GFR decline did not correlate with glomerular podocyte number, intra-renal or urinary intra-renal expression of any target gene ().
TABLE 2. Correlation between the rate of renal function decline and the glomerular podocyte number, intra-renal, and urinary gene expression
DISCUSSION
In this study, we studied intra-renal and urinary mRNA expression of podocyte-associated molecules as well as glomerular podocyte counting to estimate the podocyte injury in human diabetic nephropathy. We found that intra-renal expression of nephrin, podocin, and synaptopodin correlated with glomerular podocyte number, estimated GFR, and the degree of tubulointerstitial scarring. Urinary expression of podocyte-associated molecules, on the contrary, partly correlated with proteinuria and renal function, but not glomerular podocyte number or intra-renal expression of the corresponding molecule.
A number of studies in recent years have estimated the number of podocytes in glomeruli.Citation5,Citation10,Citation31–34 However, these results were not consistent. Many factors such as podocyte density-calculating method, glomerular volume measurement, and the way chosen to recognize podocyte nucleus might result in these discrepancies. Serial sectioning throughout glomeruli is usually needed to get an accurate estimation of glomerular podocyte number.Citation31,Citation32 The technique, however, is time consuming and usually not applicable in clinical setting because it requires a large amount of renal tissue. Since there are many molecules specifically expressed by podocyte, it is possible that their expression levels in glomeruli or urine reflect podocyte loss.Citation35 From an anatomical point of view, reduction in the expression of molecules that belong to foot process and slit diaphragm may destabilize coherence between podocytes and basement membrane leading to podocyte loss. The correlations between intra-renal expression of podocyte-associated molecules and glomerular podocyte number observed in this study suggest that measurement of intra-renal expression of podocyte-associated molecules may replace podocyte counting as the method to quantify podocyte injury in patients with diabetic nephropathy kidney and possibly other kidney diseases.
Urinary expression of podocyte-associated molecules, in contrast, did not correlate with glomerular podocyte number. The significant correlation between urinary synaptopodin expression and glomerular podocyte number was possibly false positive as a result of multiple statistical comparison. Taken together, urinary expression of these markers cannot be used to estimate glomerular podocyte number. In addition, we did not find any correlation between urinary and intra-renal expression of targeted genes. The discrepancy between urinary expression of podocyte-associated molecules and intra-renal gene expression or podocyte count suggests that apoptosis, rather than shedding into urine, is the major route of podocyte loss in diabetic nephropathy. In fact, glucose-induced reactive oxygen species has been shown to cause apoptosis of podocytes at the onset of diabetic nephropathy.Citation36 Podocyte detachment, however, may be a major cause of podocyte loss in other kidney diseases – particularly glomerulonephritis.Citation18 The use of urinary gene expression in these diseases needs further study.
In line with the current notion that podocyte loss causes focal sclerosis,Citation7,Citation37 we found close correlations between podocyte-associated molecules and tubulointerstitial scarring. Unlike the findings by other groups,Citation4,Citation35 however, we did not observe a correlation between proteinuria and podocyte number or intra-renal nephrin expression. This discrepancy may be a result of the relatively small sample size in our study, or possible selection bias – diabetic patients usually do not need renal biopsy unless there are atypical feature. We also did not observe any correlation between gene expression and GFR decline rate, probably also because of the small sample size. Unfortunately, we could not further increase the sample size of this study because of the limited number of patients with diabetic nephropathy undergoing renal biopsy. It is also important to recognize that gene expression level probably varies at different stages of diabetic nephropathy. Since we focused on moderate to advanced stage of disease in this study, the result could be different from other reports on earlier stages of disease. Further prospective studies with larger sample size and probably longer follow-up are needed to determine the role of intra-renal expression of podocyte-associated molecules as a predictor of renal function decline.
In summary, we found that intra-renal expression of podocyte-associated molecules correlated with glomerular podocyte number, as well as the estimated GFR and the degree of tubulointerstitial scarring. In contrast, urinary mRNA expression of podocyte-associated molecules did not correlate with glomerular podocyte number or intra-renal expression of the corresponding molecule. The results suggest that intra-renal expression of podocyte-associated molecules could be used as a surrogate marker to assess podocyte loss in diabetic nephropathy.
Acknowledgments
This study was supported by the Hong Kong Society of Nephrology research grant and CUHK research account 6901031. We thank Professor John M. Basgen for his useful advice on podocyte counting, and Professor Chan Ho-Yin and Dr Law Tik-wan from the Department of Biochemistry, Chinese University of Hong Kong, for the technical support.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this paper.
REFERENCES
- Cooper L. USRDS annual data report. Nephrol News Issues. 2001;15:31–38.
- Dagogo-Jack S. Primary prevention of type-2 diabetes in developing countries. J Natl Med Assoc. 2006;98:415–419.
- Marshall SM. The podocyte: A major player in the development of diabetic nephropathy? Horm Metab Res. 2005;37:9–16.
- White KE, Bilous RW, Diabiopsies study G. Structural alterations to the podocyte are related to proteinuria in type 2 diabetic patients. Nephrol Dial Transplant. 2004;19:1437–1440.
- White KE, Bilous RW, Marshall SM, Podocyte number in normotensive type 1 diabetic patients with albuminuria. Diabetes. 2002;51:3083–3089.
- Macconi D, Bonomelli M, Benigni A, Pathophysiologic implications of reduced podocyte number in a rat model of progressive glomerular injury. Am J Pathol. 2006;168:42–54.
- Kriz W, Lemley KV. The role of the podocyte in glomerulosclerosis. Curr Opin Nephrol Hypertens. 1999;8:489–497.
- Pagtalunan ME, Miller PL, Jumping-Eagle S, Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Invest. 1997;99:342–348.
- Shirato I, Hishiki T, Tomino Y. Podocyte loss and progression of diabetic nephropathy. Contrib Nephrol. 2001;134:69–73.
- Dalla Vestra M, Masiero A, Roiter AM, Saller A, Crepaldi G, Fioretto P. Is podocyte injury relevant in diabetic nephropathy? Studies in patients with type 2 diabetes. Diabetes. 2003;52:1031–1035.
- Mundel P, Shankland SJ. Podocyte biology and response to injury. J Am Soc Nephrol. 2002;13:3005–3015.
- Petermann AT, Pippin J, Krofft R, Viable podocytes detach in experimental diabetic nephropathy: Potential mechanism underlying glomerulosclerosis. Nephron Exp Nephrol. 2004;98:e114–e123.
- Petermann AT, Krofft R, Blonski M, Podocytes that detach in experimental membranous nephropathy are viable [see comment]. Kidney Int. 2003;64:1222–1231.
- Vogelmann SU, Nelson WJ, Myers BD, Lemley KV. Urinary excretion of viable podocytes in health and renal disease. Am J Physiol Renal Physiol. 2003;285:F40–F48.
- Camici M. Urinary detection of podocyte injury. Biomed Pharmacother. 2007;61:245–249.
- Hara M, Yanagihara T, Itoh M, Matsuno M, Kihara I. Immunohistochemical and urinary markers of podocyte injury. Pediatr Nephrol. 1998;12:43–48.
- Patari A, Forsblom C, Havana M, Taipale H, Groop PH, Holthofer H. Nephrinuria in diabetic nephropathy of type 1 diabetes. Diabetes. 2003;52:2969–2974.
- Yu D, Petermann A, Kunter U, Rong S, Shankland SJ, Floege J. Urinary podocyte loss is a more specific marker of ongoing glomerular damage than proteinuria. J Am Soc Nephrol. 2005;16:1733–1741.
- Wang G, Lai F-M, Lai K-B, Chow K-M, Li K-TP, Szeto C-C. Messenger RNA expression of podocyte-associated molecules in the urinary sediment of patients with diabetic nephropathy. Nephron Clin Pract. 2007;106:c169–c179.
- Szeto CC, Lai KB, Chow KM, Messenger RNA expression of glomerular podocyte markers in the urinary sediment of acquired proteinuric diseases. Clin Chim Acta. 2005;361:182–190.
- Wirta O, Helin H, Mustonen J, Kuittinen E, Savela T, Pasternack A. Renal findings and glomerular pathology in diabetic subjects. Nephron. 2000;84:236–242.
- Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group [see comment]. Ann Intern Med. 1999;130:461–470.
- Weibel ER. Stereological Methods. Practical Methods for Biological Morphometry. Vol 1. London: Academic Press, Inc; 1979:40–116.
- Barisoni L, Kriz W, Mundel P, D'Agati V. The dysregulated podocyte phenotype: A novel concept in the pathogenesis of collapsing idiopathic focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol. 1999;10:51–61.
- Najafian B, Basgen JM, Mauer M. Estimating mean glomerular volume using two arbitrary parallel sections. J Am Soc Nephrol. 2002;13:2697–2705.
- Bruneval P, Bariety J, Belair MF, Mandet C, Heudes D, Nicoletti A. Mesangial expansion associated with glomerular endothelial cell activation and macrophage recruitment is developing in hyperlipidaemic apoE null mice. Nephrol Dial Transplant. 2002;17:2099–2107.
- Li B, Hartono C, Ding R, Noninvasive diagnosis of renal-allograft rejection by measurement of messenger RNA for perforin and granzyme B in urine. N Engl J Med. 2001;344:947–954.
- Szeto CC, Chan RW, Lai KB, Messenger RNA expression of target genes in the urinary sediment of patients with chronic kidney diseases. Nephrol Dial Transplant. 2005;20:105–113.
- Biosystems FCCA. Guide to Performing Relative Quantitation of Gene Expression Using Real-Time Quantitative PCR. 2004.
- Biosystems FCCA. Amplification Efficiency of TaqMan® Gene Expression Assays. 2006.
- Basgen JM, Nicholas SB, Mauer M, Rozen S. Nyengaard JR. Comparison of methods for counting cells in the mouse glomerulus. Nephron Exp Nephrol. 2006;103:e139–e148.
- White KE, Bilous RW. Estimation of podocyte number: A comparison of methods. Kidney Int. 2004;66:663–667.
- Siu B, Saha J, Smoyer WE, Sullivan KA, Brosius FC3rd. Reduction in podocyte density as a pathologic feature in early diabetic nephropathy in rodents: Prevention by lipoic acid treatment. BMC Nephrol. 2006;7:6.
- Sanden SK, Wiggins JE, Goyal M, Riggs LK, Wiggins RC. Evaluation of a thick and thin section method for estimation of podocyte number, glomerular volume, and glomerular volume per podocyte in rat kidney with Wilms’ Tumor-1 protein used as a podocyte nuclear marker. J Am Soc Nephrol. 2003;14:2484–2493.
- Langham RG, Kelly DJ, Cox AJ, Proteinuria and the expression of the podocyte slit diaphragm protein, nephrin, in diabetic nephropathy: Effects of angiotensin converting enzyme inhibition. Diabetologia. 2002;45:1572–1576.
- Susztak K, Raff AC, Schiffer M, Böttinger EP. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes. 2006;55:225–233.
- Kretzler M. Role of podocytes in focal sclerosis: Defining the point of no return [comment]. J Am Soc Nephrol. 2005;16:2830–2832.