Abstract
Background: Chronic inflammation and oxidative stress are prevalent in hemodialysis (HD) patients. We evaluated the long-term effect of a vitamin E-coated cellulose acetate (CAE) membrane on oxidative stress and inflammation. Methods: Nine patients were switched to CAE membrane for 3 months and then changed back to polysulfone (PS) membrane again for 6 months. Reactive oxygen metabolites and derivatives (d-ROMs), total antioxidant capacity (TAC) and superoxide dismutase (SOD) (oxidative stress biomarkers), high-sensitivity C-reactive protein (Hs-CRP), and interleukin-6 (IL-6) (inflammation biomarkers) were measured. Results: d-ROMs decreased and TAC rose significantly at the end of the study, whereas SOD increased rapidly and immediately after the end of CAE treatment. Hs-CRP and IL-6 levels were significantly lowered at the end of the study. Conclusions: Vitamin E supplementation by vitamin E-coated CAE dialysis membrane suppresses oxidative stress and inflammation.
INTRODUCTION
In dialysis patients, an increase in oxidative and chronic inflammatory state are prominent features.Citation1 These conditions contribute to endothelial dysfunction and accelerated atherosclerosis and finally to the high incidence of cardiovascular disease, the single most common cause of excess morbidity and mortality in the end-stage renal disease (ESRD) patients undergoing dialysis treatment.Citation2,Citation3 The causes of inflammation include both factors arising from dialysis itself (efficiency and biocompatibility issues) as well as other nondialysis-related factors such as advanced age, diabetes, renal disease, and uremia per se.Citation4 ESRD patients have increased levels of inflammation-related proteins, such as interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), and C-reactive protein (CRP), and are subjected to enhanced oxidative stress, as a result of both insufficient antioxidant defense mechanisms and excessive generation of oxidant compounds.Citation3,Citation5 Despite rapid improvements in dialysis technology during the last 20 years, the mortality rate in ESRD patients treated with dialysis has remained unacceptably high; therefore, studies on the effects of various anti-inflammatory treatment strategies are warranted to improve outcomes in this patient population.Citation4
The use of vitamin E-coated dialysis membranes (a multilayer membrane with liposoluble vitamin E on the blood surface allowing direct free radical scavenging at the membrane site) exhibited antioxidant effects as evaluated by a decrease in the serum levels of several markers of oxidative stress (malonyldialdehyde, advanced glycation endproducts (AGEs), and 8-hydroxydeoxyguanosine) in 18 prevalent hemodialysis (HD) patients treated with this filter for 6 months.Citation6 Another study demonstrated that treatment with vitamin E-coated polysulfone (PS) membrane for 6 months reduced predialysis levels of asymmetric dimethylarginine (ADMA), a marker of oxidative stress and endothelial dysfunction, in HD patients.Citation7 Furthermore, vitamin E-coated dialyzer membrane appeared to downregulate the expression of monocytes adhesion molecules, as demonstrated in a prospective cross-over trial in 14 stable HD patients.Citation8 However, when vitamin E-coated membranes were recently compared with biocompatible PS membrane, there was no difference of oxidative stress parameters.Citation9 Thus, the biocompatibility advantage of vitamin E-coated membranes remains unsettled.
So, this study was designed to elucidate prospectively the long-term effects of a vitamin E-coated dialysis membrane on both oxidative stress and inflammation, compared to PS membrane, in a group of stable patients on regular HD.
METHODS AND PATIENTS
We performed a prospective study in a group of nine stable HD patients aged 50–71 years (median age 66 years; six men, three women, two with polycystic disease, and one with nephrosclerosis and six with unknown origin) on dialysis since 8–142 months (median duration 52 months). The median dialysis time per week was 12 h (9–15). Diabetic patients and patients on angiotensin-converting enzyme (ACE) inhibitors, statins, corticosteroids, or with evidence of inflammatory disease were excluded. The hematology, biochemical, and treatment parameters are shown in .
TABLE 1. Hematology, biochemical, and treatment parameters
At inclusion, all patients were dialyzed three times a week with low-flux PS membrane for 12 h/week (9–15). All patients were switched to vitamin E-coated cellulose acetate (CAE) membrane (Terumo 18EE, 1.8 m2, Terumo Inc., Tokyo, Japan) for 3 months and then changed back to their previous dialysis membrane for 6 months. All patients underwent HD with low-molecular-weight heparins and were on carnitine, sevelamer, vitamin B complex, folic acid, iron sucrose IV 100–200 μg/month (Venofer®), and erythropoietin (targeting Hb 11–13 g/dL). All dialysis parameters, except dialyzers, were kept unchanged throughout the study. The study was approved by the hospital's ethics committee. All patients gave a written informed consent.
The oxidative stress status was assessed by the determination of reactive oxygen metabolites and derivatives (d-ROMs), total antioxidant capacity (TAC), and superoxide dismutase (SOD). The inflammation status was evaluated by high-sensitivity CRP (Hs-CRP) and IL-6.
Oxidative stress biomarkers
TAC was measured spectrophotometrically (Hitachi U-1800 UV–Vis spectrophotometer). Briefly, TAC was determined using the manual method of Benzie and Strain,Citation10 which measures the reduction of ferric to ferrous ion in the presence of antioxidants [ferric reducing ability of plasma (FRAP) assay].
The final result was expressed as μM of ferrous sulfate. The FRAP assay was performed in triplicate on each sample and the mean of the three values was used in the analysis. The intra-assay and the inter-assay coefficients of variation were 3.5 and 10.2%, respectively.
d-ROMs were evaluated spectrophotometrically by the d-ROMs Test™-FRAS 4, Grosseto, Italy. This test is based on the ability of transition metals to catalyze, in the presence of peroxides, the formation of free radicals that are then trapped by an alkylamine. The alkylamine reacts forming a colored radical detectable at 505 nm through a kinetic reaction that is linear up to 500 U CARR (Carratelli Units). The determination of free radicals can be made with a normal spectrophotometer. The normal range has been determined as 250–300 U CARR.Citation11
SOD activity was determined using commercially available determination kit (Fluka Chemie GmbH, Buchs, Switzerland). The kit allows SOD assaying based on the capacity of SOD or SOD-like materials to inhibit the reduction of water-soluble tetrazolium salt to a water-soluble formazan dye by the xanthine–xanthine oxidase system. The development of the reaction was monitored spectrophotometrically at 450 nm using a microplate reader (Expert 96, ASYS Hitech, Eugendorf, Austria). One SOD unit was defined as the amount of enzyme that inhibited the reduction of cytochrome c by 50% in a coupled system with xanthine oxidase at pH 7.8 at 25°C in a 3.0 mL reaction volume. The final result was expressed as SOD unit per milliliter. The SOD assay was performed in duplicate on each sample and the mean of the two values was used in the analysis. The intra-assay and the inter-assay coefficients of variation were 4.7 and 7.9%, respectively.
Inflammatory biomarkers
Serum Hs-CRP and IL-6 levels were measured using commercially available enzyme-linked immunosorbent assay.
Hs-CRP assay uses a kit specific for human CRP (Bender MedSystems, Vienna, Austria) according to the manufacturer's instructions. The CRP assay was performed in duplicate on each sample and the mean of the two values was used in the statistical analysis. The results are expressed in milligram per liter of sample. The sensitivity of the technique allows the detection levels as low as alkylamine 3.0 pg/mL. The intra-assay and inter assay coefficients of variation were 3.8 and 4.7%, respectively.
IL-6 levels were measured using sandwich ELISA kits specific for human IL-6 (BioMedix IVD, Athens, Greece) according to the manufacturer's instructions. The assays were performed in duplicate on each sample and the mean of the two values was used in the statistical analysis. The results are expressed in picograms per milliliter of sample. The sensitivity of the technique allows the detection of levels as low as 1.6 pg/mL for IL-6. The intra-assay and inter-assay coefficients of variation were 5.5 and 7.6% for IL-6.
Blood sampling
After overnight fasting, blood samples were drawn from AV fistulas before the second HD session of the week. Blood samples were collected at baseline, 3, and 9 months. Samples were immediately centrifuged and the plasma was stored at −80°C until analysis. Iron supplementation was at least 2 weeks away from blood sampling for the determination of inflammatory and oxidative stress biomarkers.
Statistics
Data are presented as mean ± SD. Nonparametric tests (Wilcoxon-matched pairs and Mann–Whitney unpaired tests) were applied for statistical analysis. Statistical significance was set at p < 0.05
RESULTS
Biochemical and hematology parameters
KT/V urea, estimated by Daugirdas formula, remained stable throughout the study. No significant variations in Hb, white cell and platelet counts, total protein, albumin, total cholesterol, triglycerides, high-density lipoprotein (HDL), low-density lipoprotein (LDL), parathyroid hormone (PTH), and uric acid were observed throughout the study.
Oxidative stress biomarkers
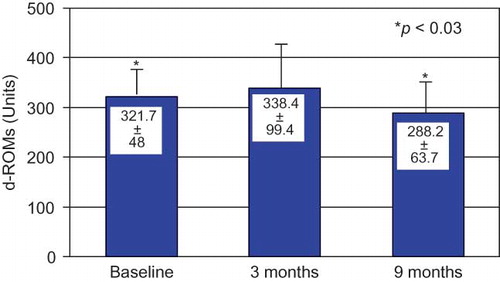
d-ROMs levels showed a significant reduction 6 months after the end of CAE membrane use ().
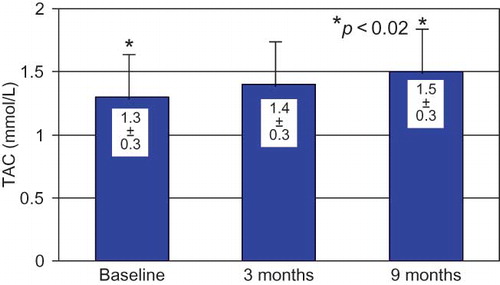
TAC levels were increased in comparison to baseline values under CAE dialysis and this increase became significant by the end of 9 months of the study ().
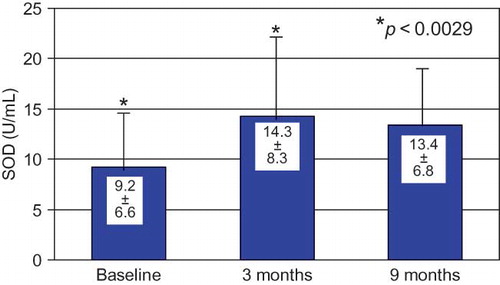
SOD activity was significantly increased on the third month of CAE membrane use and the levels remained stable through the end of the study (month 9) ().
Inflammatory biomarkers
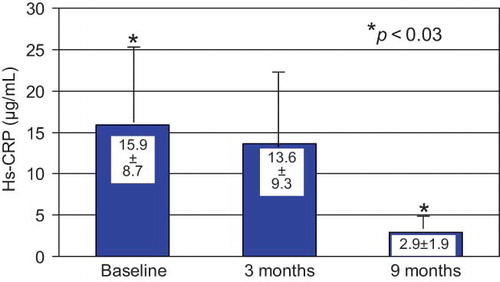
A decrease in plasma Hs-CRP levels was observed after CAE membrane use that became significant by the end of the study (month 9) ().
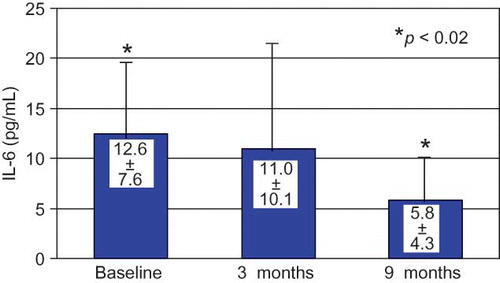
IL-6 levels decreased by the end of CAE membrane use and this decrease was significant at 9 months ().
DISCUSSION
Oxidative stress is highly prevalent in dialysis patients. It is incriminated in the pathogenesis of anemia and cardiovascular disease increasing morbidity and mortality in ESRD.Citation4,Citation12 Interaction between dialysis membrane and circulating blood neutrophils results in the release of oxygen-free radicals and oxidizing agents such as superoxide anions, hydrogen peroxides, and myeloperoxidase.Citation13
In the general population, inflammation, as evidenced by CRP levels, is predictive of mortality from cardiovascular disease. CRP elevation is related to earlier changes such as elevated levels of IL-6 which may be a proxy for other inflammatory variables in this case.Citation14–17 The potential synergy between oxidative stress and inflammation is of particular interest because inflammation is a major issue in long-term morbidity and mortality dialysis.Citation18–20 Several studies reported that oxidative stress and inflammation might be linked to dialysis patients. The presence of inflammation and the duration of dialysis could be the most important factors in oxidative stress generation in this population.Citation21,Citation22 Consequently, antioxidative treatment might be beneficial in decreasing inflammation in ESRD. Short-term administration of γ-enriched tocopherols led to a decrease in median CRP level in HD patients as reported by Himmelfarb et al.Citation23
Chronic inflammation markers are frequently increased in dialysis patients.Citation18,Citation19,Citation24,Citation25 Oxidative stress is an important component of the inflammatory process.Citation26 It has been suggested that part of the oxidative stress found in chronic inflammation results from reactive oxygen species released by dialysis-activated white cells.Citation27
Vitamin E is the most effective chain-breaking lipid-soluble antioxidant in biological membranes; it contributes to the stability of the cell membrane and protects the cell from damage caused by oxygen-free radicals and lipid peroxidation products. In an attempt to directly scavenge free radicals, the use of oral vitamin E supplementation has been proposed as a therapeutic strategy. This can also be performed by coating vitamin E to the dialysis membrane. The CAE membrane can exert a site-specific and timely scavenging function against oxidation-free radicals in synergy with its effect in decreasing leukocyte activation and oxidation-free radical production.Citation28 Conflicting results are reported on the oxidative stress as well as on the inflammation biomarkers generation during dialysis session with vitamin E-coated membranes.Citation29–33
Proof of the relationship between inflammation marker CRP and oxidative stress is important because of the prominent role for CRP in predicting outcomes in dialysis patients.Citation24,Citation25,Citation34. As far as it concerns patients with kidney disease, there is no consensus approach to assess their inflammatory state. In chronic kidney disease (CKD), inflammation markers such as ferritin (positive acute-phase protein) and albumin (negative acute-phase protein) have been widely used. Serum ferritin levels are increased in malnutrition, in iron supplementation as well as in inflammatory states.Citation35 Albumin is the predominant oxidatively modified plasma protein in patients on HD and oxidation of albumin will decrease plasma antioxidant defenses. However, factors other than the catabolic consequences of inflammation also affect serum albumin, in particular nutritional status.Citation36 Markers like albumin are not reliable indicators of inflammatory status, explaining the nonsignificant variation of these parameters observed in our study. Iron supplementation can explain the increase in ferritin levels.
Other clinical indicators of inflammatory status, such as erythropoietin dose for anemia correction and WBC count, were not found significantly modified under CAE membrane dialysis. Our data indicate a beneficial long-term effect of CAE, as opposed to PS membrane, on inflammation by a significant decrease in CRP levels, a more reliable inflammatory biomarker of acute-phase reaction, especially when measured with a high-sensitivity assay as it was the case in our study.Citation37 CRP levels showed a significant decrease by the end of our study a long time after the end of CAE membrane use.
Overproduction of proinflammatory cytokines, such as IL-1β, IL-6, and TNF-α, is also well documented in dialysis patients.Citation28,Citation30,Citation31 Our study reports a decrease in IL-6 by the end of CAE dialysis period, which became significant 6 months later, by the end of the study. Our results on Hs-CRP and IL-6 levels suggest a long-lasting effect of the vitamin E-modified membrane on the patients' inflammation status and this is, to our knowledge, the first reported evidence of such a prolonged effect.
SOD, catalase (CAT), and the glutathione (GSH) system provide most of the protection against damage by oxygen radicals. We report a significant increase in SOD levels rapidly and immediately after the 3-month CAE membrane period is reported.
A Japanese study indicated that a vitamin E-coated regenerated cellulose dialyzer used for 6 months is more effective than a PS dialyzer in diminishing oxidative stress to all biomolecules, including lipids, proteins, and nucleic acids during HD especially in DM patients.Citation6 In our study, using different markers, we also reported that CAE membrane diminishes oxidative stress in the long term.
d-ROMs levels, which are found higher in dialysis patients and exhibit a further increase during the dialysis procedure, are the oxidative stress biomarkers more accurately reflecting dialysis oxidative alterations. We report a significant reduction in d-ROMs levels in our dialysis patients that are maintained long after the end of CAE membrane use. In accordance with our findings is another long-term study which has included 31 HD patients and has demonstrated that biocompatible PS membranes coated with vitamin E reduces oxidative stress using the ADMA as an oxidative stress marker.Citation7
TAC determination in dialysis patients is severely affected by the concomitant fluctuation in plasma urate levels and therefore must be carefully interpreted. However, in our study, plasma urate levels remained stable. The FRAP method for TAC determination, which we used, represents the most sensitive index of plasma antioxidant capacity according to a recent study evaluating the biomarkers of oxidative stress in this population.Citation38 Our patients presented a significant increase in TAC under CAE membrane use lasting to the end of the study. Our findings are in accordance with another short-term study of eight hemodialyzed patients, where the modified dialyzer with vitamin E led to a significant increase of TAC.Citation39 Also Mydlick et al., in a previous short-term study (3 months), have reported the beneficial effect of the vitamin E-coated dialyzer against oxidative stress in 14 HD patients where erythrocyte (ER) antioxidant enzymes, SOD, GSH peroxidase (GPX), GSH reductase (GR), and CAT, plasma TAC, RBC GSH, plasma malondialdehyde (MDA), plasma, and RBC vitamin E were investigated.Citation30 Our study is a pioneer study because it has demonstrated not only the beneficial effects of CAE membrane on oxidation as the above studies have but also has reported for the first time the extended duration of these effects.
A relevant limitation in our methodology is the nonrandomized and uncontrolled nature of our trial. However, this is, to our knowledge, a pioneering study on the long-term impact of CAE membranes on both inflammation and oxidative stress. On this issue, data from research and clinical trials are scanty, even on the short-term effects. To the opposite, the use of multiple reliable and well-established markers of inflammation and oxidative stress as those applied in our study strengthens our conclusions. The results of this study could serve as a working hypothesis for further studies with larger numbers of patients.
Oxidative stress markers, such as SOD, responded promptly and significantly to the treatment intervention whereas d-ROMs and TAC were delayed in achieving significance. This could be due to various reasons such as our study's small number of patients and differences in our markers sensitivity. Another speculated reason could be that the result is not statistically significant from the beginning, therefore in the first 3 months of the study, but later. Another speculated reason could be that the result is not statistically significant from the beginning therefore in the first 3 months of the study.
Inflammation biomarkers decreased slowly to a significant level, long after vitamin E-associated dialysis membrane treatment. As other reports validating our findings are not available, we cannot conclude on the significance of this timing of oxidative stress responses until confirmatory evidence is published. We could only speculate the same reasons we reported for the oxidative stress markers, which are the small number of enrolled patients, the duration of the study, the possible positive result of released vitamin E in the plasma after the 3 months.
In conclusion the results of this study indicate that CAE is more effective than the common PS dialyzer in diminishing the oxidative stress as well as the inflammation in dialysis patient. A link of oxidative stress and inflammation is also suggested by our findings as antioxidant biomarkers responded first to vitamin E-associated dialysis membrane use while the effect on inflammation biomarkers followed, which can possibly be explained by an association between inflammation and oxidative stress. Our results could serve as a useful working hypothesis for further studies with a larger number of patients as well as for extended study duration to elucidate the potential benefits of these CAE on oxidative stress/inflammation-related morbidity and mortality and to conclusively determine the related factors.
Acknowledgments
This work was presented in abstract form in the XLIV Congress of the European Renal Association-European Dialysis and Transplantation Association, Barcelona, Spain, 21–24 June 2007.
The authors thank all the renal nurses involved in recruitment participants and everyone who took part in the study
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this paper.
REFERENCES
- Kaysen GA. The microinflammatory state in uremia: Causes and potential consequences. J Am Soc Nephrol. 2001;12:1549–1557.
- Rayner HC, Pisoni RL, Bommer J, Mortality and hospitalization in hemodialysis patients in five European countries: Results from the dialysis outcomes and practice patterns study (DOPPS). Nephrol Dial Transplant. 2004;19:108–120.
- Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305.
- Locatelli F, Canaud B, Eckardt KU, Stenvinkel P, Wanner C, Zoccali C. Oxidative stress in end-stage renal disease: An emerging threat to patient outcome. Nephrol Dial Transplant. 2003;18:1272–1280.
- Spittle MA, Hoenich NA, Handelman GJ, Adhikarla R, Homel P, Levin NW. Oxidative stress and inflammation in hemodialysis patients. Am J Kidney Dis. 2001;38:1408.
- Satoh M, Yamasaki Y, Nagake Y, Oxidative stress is reduced by the long-term use of vitamin E-coated dialysis filters. Kidney Int. 2001;59:1943–1950.
- Morimoto H, Nakao K, Fukuoka K, Long-term use of vitamin E-coated polysulphone membrane reduces oxidative stress markers in hemodialysis patients. Nephrol Dial Transplant. 2005;20:2775–2782.
- Betjes MG, Hoekstra FM, Klepper M, Postma SM, Vaessen LM. Vitamin E-coated dialyzer membranes downregulate expression of monocytes adhesion and co-stimulatory molecules. Blood Purif. 2004;22(6):510–517.
- Morena M, Gausson V, Mothu N, Regional variations of low-density lipoprotein oxidizability in hemodialysis patients may explain discrepancies in interventional therapy on oxidative profile. Blood Purif. 2008;26:300–310.
- Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal Biochem. 1996;239:70–76.
- Cesarone MR, Belgaro G, Carratelli M, A simple test to monitor oxidative stress. Int Angiol. 1999;18:127–130.
- Himmelfarb J, Stenvinkel P, Ikizler TA, Hakim RM. The elephant in uremia: Oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int. 2002;62:1524–1538.
- Jaber BL, Pereira BJ. Biocompatibility of hemodialysis membranes. In: Pereira B, Sayegh M, Blake P, eds. Chronic Kidney Disease, Dialysis and Transplantation: A Companion to Brenner and Rector's The Kidney. 2nd ed. Philadelphia, PA: Elsevier Saunders; 2005:363–387.
- Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973–979.
- Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843.
- Owen WF, Lowrie EG. C-reactive protein as an outcome predictor for maintenance hemodialysis patients. Kidney Int. 1998;54:627–663.
- Weinhold B, Bader A, Poli V, Ruther U. Interleukin-6 is necessary, but not sufficient, for induction of the human C-reactive protein gene in vivo. Biochem J. 1997;325:617–621.
- Lowrie EG. Acute-phase inflammatory process contributes to malnutrition, anemia, and possibly other abnormalities in dialysis patients. Am J Kidney Dis. 1998;32:S105–S112.
- Bistrian BR. Role of the systemic inflammatory response syndrome in the development of protein-calorie malnutrition in ESRD. Am J Kidney Dis. 1998;32:S113–S117.
- Kopple JD. Pathophysiology of protein-energy wasting in chronic renal failure. J Nutr. 1999;129:247S–251S.
- Nguyen-Khoa T, Massy ZA, De Bandt JP, Oxidative stress and hemodialysis: Role of inflammation and duration of dialysis treatment. Nephrol Dial Transplant. 2001;16:335–340.
- Handelman GJ, Walter MF, Adhikarla R, Elevated plasma F2-isoprostanes in patients on long-term hemodialysis. Kidney Int. 2001;59:1960–1966.
- Himmelfarb J, Kane J, McMonagle E, Alpha and gamma tocopherol metabolism in healthy subjects and patients with end-stage renal disease. Kidney Int. 2003;64:978–991.
- Iseki K, Tozawa M, Yoshi S, Fuyikama K. Serum C-reactive protein (CRP) and risk of death in chronic dialysis patients. Nephrol Dial Transplant. 1999;14:1956–1960.
- Yeun JY, Levine RA, Mantadilok V, Kaysen GA. C-reactive protein predicts all-cause and cardiovascular mortality in hemodialysis patients. Am J Kidney Dis. 2000;35:469–476.
- Hensley K, Robinson KA, Gabbita SP, Salsman S, Floyd RA. Reactive oxygen species, cell signaling, and cell injury. Free Radic Biol Med. 2000;28:1456–1462.
- Rosen GM, Pou S, Ramos CL, Cohen MS, Britigan BE. Free radicals and phagocytic cells. FASEB J. 1995;9:200–209.
- Girndt M, Lengler S, Kaul H, Sester U, Sester M, Köhler H. Prospective crossover trial of the influence of vitamin E-coated dialyzer membranes on T-cell activation and cytokine induction. Am J Kidney Dis. 2000;35(1):95–104.
- Azzi A, Boscoboinik D, Hensey C. The protein kinase C family. Eur J Biochem. 1992;208(3):547–557.
- Mydlík M, Derzsiová K, Rácz O, Vitamin E-coated dialyzer and antioxidant defense parameters: Three-month study. Semin Nephrol. 2004;24(5):525–531.
- Durak I, Akyol O, Başeşme E, Canbolat O, Kavutçu M. Reduced erythrocyte defense mechanisms against free radical toxicity in patients with chronic renal failure. Nephron. 1994;66(1):76–80.
- Ceballos-Picot I, Witko-Sarsat V, Merad-Boudia M, Glutathione antioxidant system as a marker of oxidative stress in chronic renal failure. Free Radic Biol Med. 1996;21(6):845–853.
- Boran M, Küçükaksu C, Balk M, Cetin S. Red cell lipid peroxidation and antioxidant system in hemodialysed patients: Influence of recombinant human erythropoietin (r-HuEPO) treatment. Int Urol Nephrol. 1998;30(4):507–512.
- Zimmermann J, Herrlinger S, Pruy A, Metzeger T, Wanner C. Inflammation enhances cardiovascular risk and mortality in hemodialysis patients. Kidney Int. 1999;55:648–658.
- Honda H, Qureshi AR, Heimbürger O, Serum albumin, C-reactive protein, interleukin 6 and Fetuin A as predictors of malnutrition, cardiovascular disease and mortality in ESRD patients. Am J Kidney Dis. 2006;47:139–148.
- Senol E, Ersoy A, Erdinc S, Sarandol E, Yurtkuran M. Oxidative stress and ferritin levels in hemodialysis patients. Nephrol Dial Transplant. 2008;23:665–672.
- Menon V, Greene T, Wang X, C-reactive protein and albumin as predictors of all-cause and cardiovascular mortality in chronic kidney disease. Kidney Int. 2005;68:766–772.
- Paul JL, Sall ND, Soni T, Lipid peroxidation abnormalities in hemodialyzed patients. Nephron. 1993;64(1):106–109.
- Mydlík M, Derzsiová K, Rácz O, A modified dialyzer with vitamin E and antioxidant defense parameter. Kidney Int Suppl. 2001;78:S144–S147.