Abstract
Gitelman's syndrome, or congenital hypokalemic hypomagnesemic hypocalciuria with metabolic alkalosis, is widely described as a benign or milder variant of Bartter's syndrome and most commonly presents with transient periods of weakness and fatigue, presyncope, vertigo, ataxia, and blurred vision, though aborted sudden cardiac death has also been rarely reported. Despite this there are limited data in the literature regarding the formal cardiac evaluation of patients with Gitelman's syndrome. We present the case of a gentleman with Gitelman's syndrome who initially presented to his primary physician with symptoms suggestive of an upper respiratory tract infection and subsequently survived a ventricular fibrillation (VF) cardiac arrest in the community. We review the literature regarding possible life-threatening cardiac complications in these patients.
INTRODUCTION
Gitelman's syndrome is an inherited renal tubulopathy transmitted in an autosomal recessive manner. It results from mutations in the solute carrier family 12, member 3 (SLC12A3) gene encoding for the thiazide-sensitive sodium chloride cotransporter in the distal convoluted tubules, leading to hypokalemia, hypomagnesaemia, alkalosis, and reduced urinary calcium excretion rates.Citation1 Patients with Gitelman's syndrome are often asymptomatic but may also present with transient periods of weakness and fatigue, presyncope, vertigo, ataxia, and blurred vision.Citation2 Aborted sudden cardiac death (SCD) has also rarely been reported in patients with Gitelman's syndrome.Citation3 Malignant ventricular tachyarrhythmias are believed to be precipitated by the associated hypokalemia and hypomagnesaemia which cause prolongation of the ventricular action potential and which may be manifested by a lengthening of the QT interval on the surface electrocardiogram (ECG).Citation4–6 Despite this there are limited data in the literature regarding the formal cardiac evaluation of patients with Gitelman's syndrome.
We report the case of a man with Gitelman's syndrome who presented to his general practitioner (GP) with a sore throat and subsequently survived a cardiac arrest in community.
CASE REPORT
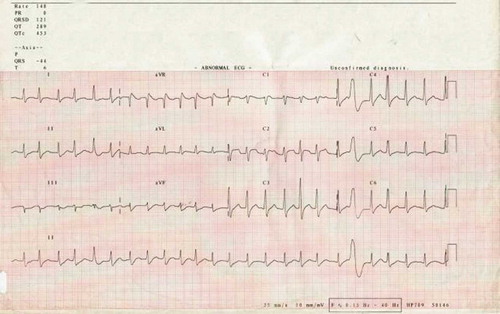
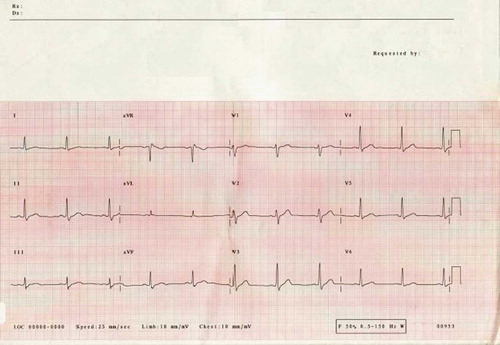
A 27-year-old man presented to his GP with a sore throat and fever. There was evidence of tonsillitis on examination and, of note, an absence of gastrointestinal symptoms or signs. While in the surgery, he suddenly collapsed and was found to be in ventricular fibrillation (VF). He was treated on site promptly with a direct current (DC) shock, successfully resuscitated, and transferred to his local hospital where he had further VF cardiac arrest and later cardioverted into atrial fibrillation (AF) (). He was intubated, ventilated, and transferred to the intensive care unit where he subsequently reverted to sinus rhythm following the administration of intravenous amiodarone (). His ECG in sinus rhythm, despite the administration of amiodarone, demonstrated a normal QT interval (QTc 436 ms), with no evidence of Brugada syndrome, arrhythmogenic right ventricular cardiomyopathy, or any ischemic changes.
His blood investigations on admission revealed the following: hemoglobin 15.4 g/dL (154 g/L) (13–18 g/dL or 130–180 g/L), white cell count 21.5 × 103/μL (4–11 × 103/μL) with a neutrophil count 17.9 × 103/μL (2–8 × 103/μL), C-reactive protein 8.5 mg/dL (< 0.5), sodium 141 mEq/L (135–145), potassium 2.4 mEq/L (3.6–5.3), magnesium 1.44 mEq/L (1.6–2.0), creatinine 0.86 mg/dL (0.70–1.3), and urea 15.9 mg/dL (7–21). His liver function tests, serum calcium, and glucose were in normal limits. Blood and throat swab cultures did not grow any organisms despite a prolonged incubation period. His urinary electrolyte levels were as follows: sodium 80.9 mEq/L, potassium 37 mEq/L, magnesium 3.4 mEq/L, and 24-hour urinary calcium 3.0 mEq (6–14 mEq/L). He underwent bedside transthoracic echocardiography which demonstrated a mildly dilated left ventricle (LV) with moderate to severely impaired systolic function (ejection fraction (EF) = 35%), and in view of the prompt treatment of his VF and very brief period of resuscitation it was assumed that his impaired LV function was of a primary rather than secondary nature to the cardiac arrest; and a presumptive diagnosis of myocarditis was made. Bloods were sent for viral titers; however, no culprit virus was subsequently identified.
He was transferred to the regional cardiac center where he made a good recovery over a 2- to 3-week period with simple supportive treatment. Subsequent echocardiography showed normalized LV systolic function. He also underwent a coronary computed tomography (CT) angiogram and cardiac magnetic resonance imaging (MRI) both of which were normal. His 24-hour Holter monitoring showed sinus rhythm with a few ventricular ectopics. An electrophysiological (EP) study for the stimulation of ventricular tachycardia (VT) was also performed during which no VT could be induced. Despite this, in view of the nature of his presentation, he was offered the insertion of an implantable cardioverter–defibrillator (ICD), which he declined. He returned to his local hospital for further recovery and rehabilitation and was discharged home 19 days after presentation. He had three further hospital admissions in the next 6 months with complaints of palpitations and dizziness believed to be secondary to the documented frequent ventricular ectopic beats. He was treated palliatively with beta blockers and his symptoms partially responded although it was noted throughout his admissions that he remained hypokalemic and hypomagnesemic despite oral supplementation of both ions. He was therefore referred to the regional nephrology unit for further investigations.
His timed urine collections showed normal cortisol and catecholamine levels. Conn's syndrome was considered unlikely as plasma renin and aldosterone levels were in normal range 12 pmol/mL/hr (0.5–5.1) and 310 pmol/L (100–800), respectively.
In view of serum and urinary electrolyte abnormalities, a diagnosis of Gitelman's syndrome was made. Genetic testing confirmed two mutations in the SLC12A3 gene consistent with Gitelman's syndrome. The patient continued treatment with potassium and magnesium supplementation in addition to a high-dose aldosterone antagonist. During the last 12 months of follow-up, he remained well with low normal serum electrolytes and occasional symptoms of palpitations. He was offered an ICD implantation on a number of occasions, which he had repeatedly declined.
DISCUSSION
Gitelman's syndrome is frequently described as a benignCitation7 or mildCitation2 variant of Bartter's syndrome. SCD has been rarely reported and is thought to be associated with QTc prolongation that is reported in around half of all patients with Gitelman's syndrome.Citation5,Citation6 This is believed to be secondary to the electrolyte imbalance or concomitant administration of QT-prolonging drugs.Citation7
Our patient presented with a VF cardiac arrest in the community after a febrile illness and had a normal QTc interval. He underwent extensive cardiac investigations and was initially considered to have myocarditis. The presence of a normal QT interval in this individual is in contrast to the previous case reports of patients with Gitelman's syndrome who have demonstrated evidence of ventricular dysrhythmias. The exact cause of his presentation, that is, a VF cardiac arrest, remains speculative although one potential mechanism suggested by the chronicity of events is that the febrile illness, on a background of profound hypokalemia and hypomagnesemia secondary to a salt-losing nephropathy, led to increased ventricular arrhythmogenicity. This may have been associated with a transient prolongation of his QT interval that was not captured on ECG before his arrest. An exaggeration of QT prolongation during fever has been shown to occur in a subset of patients with congenital long QT syndrome type 2 (LQT-2),Citation8 whereas normal individuals conversely demonstrate a shortening of the QT interval.Citation8,Citation9 Fever is also an established trigger of cardiac events, including VF, in the Brugada syndromeCitation10 in a subset of patients whose Brugada syndrome is linked to mutations in the SCN5A gene that encodes the cardiac Na+ channel. It is possibly attractive therefore to consider that a similar mechanism may underlie this event.
Other putative triggering factors for ventricular arrhythmias suggested in patients with Gitelman's syndrome have included catecholaminergic paradoxical QTc prolongation, prolongation of the QTc during nocturnal vagal stimulation, and exercise-induced LV dysfunction,Citation3 although there is no suggestion of the latter two predisposing circumstances in this case.
This report raises a number of important points for consideration; first it reaffirms the case that Gitelman's syndrome is not necessarily a benign presentation of Bartter's syndrome and can present with life-threatening arrhythmias and complications of electrolyte imbalance. Furthermore, a prolonged QTc may not be found on routine ECG and does not exclude the possibility of serious tachyarrhythmias. What are the implications of this for the physician presented with such a patient? We recommend that a thorough investigation into any potential triggering factors should be made including underlying sepsis, gastrointestinal electrolyte losses due to diarrhea and vomiting, and drugs that may either result in electrolyte imbalance or affect cardiac conduction as manifested by ECG abnormalities, particularly prolongation of the QT interval. Patients should be made aware of the need for possible lifestyle modification and risk factor avoidance to prevent consequent serious adverse events including potential SCD. In addition a systematic cardiac workup should be performed in these patients. In the case of our patient, all cardiac investigations other than the initial post-arrest echocardiogram were normal; however, in the context of a clear possible substrate for malignant ventricular arrhythmias we would recommend a comprehensive cohort of cardiac investigations to include echocardiography, cardiac MRI, serial ECGs, a minimum of 24-hour ambulatory Holter monitoring, and consideration of an electrophysiology study (VT stimulation protocol) if there are abnormalities such as frequent ventricular ectopics or a prolonged QT identified as a result of these investigations. Because Gitelman's syndrome is a rare condition, these recommendations necessarily result from a combination of our experience and the published anecdotal reports available in the literature describing the association of aborted sudden cardiac and arrhythmias with this condition.Citation3,Citation4 The lack of information available on the true cardiac risks associated with Gitelman's syndrome may be one contributory factor for our patient's continued refusal of ICD implantation. To resolve similar problems in the future, further research into the mechanisms behind these malignant arrhythmias needs to be undertaken so that specific risk stratification protocols can be developed, thus allowing targeted therapy and prevention. A relatively simple and immediate approach in the interim would be the collation of retrospective registry data of those individuals with Gitelman's syndrome to more accurately ascertain the prevalence of cardiac disorders in this group of individuals. This may also raise awareness of the potential seriousness of this disorder within both primary and secondary care settings.
Declaration of interest:The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this paper.
REFERENCES
- Bettinelli A, Vezzoli G, Colussi G, Bianchetti MG, Sereni F, Casari G. Genotype-phenotype correlations in normotensive patients with primary renal tubular hypokalemic metabolic alkalosis. J Nephrol. 1998;11:61–69.
- Cruz DN, Shaer AJ, Bia MJ, Lifton RP, Simon DB. Gitelman's syndrome revisited: An evaluation of symptoms and health-related quality of life. Kidney Int. 2001;59:710–717.
- Scognamiglio R, Negut C, Calo LA. Aborted sudden cardiac death in two patients with Bartter's/Gitelman's syndromes. Clin Nephrol. 2007;67(3):193–197.
- Pachulski RT, Lopez F, Sharaf R. Gitelman's not-so-benign syndrome. N Engl J Med. 2005;353(8):850–851.
- Foglia PE, Bettinelli A, Tosetto C, Cardiac work up in primary renal hypokalemia-hypomagnesaemia (Gitelman syndrome). Nephrol Dial Transplant. 2004;19:1398–1402.
- Bettinelli A, Tosetto C, Colussi G, Tommasini G, Edefonti A, Bianchetti MG. Electrocardiogram with prolonged QT interval in Gitelman disease. Kidney Int. 2002;62:580–584.
- Peters N, Bettinelli A, Spicher I, Basilico E, Metta MG, Bianchetti MG. Renal tubular function in children and adolescents with Gitelman's syndrome, the hypocalciuric variant of Bartter's syndrome. Nephrol Dial Transplant. 1995;10:1313–1319.
- Amin AS, Herfst LJ, Delisle BP, Fever-induced QTc prolongation and ventricular arrhythmias in individuals with type 2 congenital long QT syndrome. J Clin Invest. 2008;118(7):2552–2561.
- Karjalainen J, Viitasalo M. Fever and cardiac rhythm. Arch Intern Med. 1986;146:1169–1171.
- Keller DI, Huang H, Zhao J, A novel SCN5A mutation, F1344S, identified in a patient with Brugada syndrome and fever-induced ventricular fibrillation. Cardiovasc Res. 2006;70:521–529.