Abstract
Aim: Acute renal failure (ARF) after hematopoietic stem cell transplantation (HSCT) is a widespread complication leading to considerable morbidity and mortality. The present study aims to determine the incidence and risk factors of ARF and to investigate whether there exists a relationship between the renal injury indicators and quantity of the transplanted stem cells in a uniform patient population after allogeneic myeloablative HSCT. Methods: Patients undergoing myeloablative allogeneic HSCT from 2007 to 2008 were monitored prospectively in terms of their renal functions during the first 100 days after transplantation. ARF was defined as a twofold rise in serum creatinine concentration of baseline value or a >50% decrease in creatinine clearance and classified into three grades. Results: ARF occurred in 51.3% of patients over a period of 100 days after HSCT. ARF developed in 12 (60.0%) patients within the first 2 weeks, whereas in 8 (40.0%) of them ARF development was observed within 2–4 weeks. No correlation was found between ARF development and the quantity of the infused hematopoietic stem cells. Additionally, we were not able to identify a particular cause which was significantly associated with the occurrence of ARF after HSCT. Conclusion: A 51.3% incidence of ARF was found in patients after myeloablative allogeneic HSCT. ARF in HSCT patients could not be linked to a single cause. Rather a combination of multiple risk factors seems to be responsible for ARF development.
INTRODUCTION
Hematopoietic stem cell transplantation (HSCT) has increasingly been used as a successful therapeutic modality to cure patients with malignant hematologic disorders and also some advanced non-hematologic malignancies. However, a number of complications still limit patient survival evidently. Among those, acute renal failure (ARF) is realized to be a remarkably widespread complication leading to considerable morbidity and mortality.Citation1–4 There are several factors which can contribute to acute renal injury in this special group of patients including tumor lysis syndrome (TLS), sepsis, antibiotic nephrotoxicity, hepatic veno-occlusive disease, thrombotic microangiopathy, graft-versus-host disease (GVHD), and nephrotoxicity because of antimicrobial agents and calcineurin inhibitors.
From another point of view, stem cells in general are believed to have high tissue-regenerating capacity and their restorative potential for renal repair has been shown by a number of recent studies.Citation5–8 Renoprotective activity of already known stem cell lineages is recognized not only in ischemically injured kidneysCitation5,Citation7,Citation8 but also in cisplatin-induced toxicity.Citation9 Considering this highly regenerating and self-renewing ability of stem cells, the dilemma of high ARF rates seems somewhat surprising.
The high incidence of ARF after HSCT was mostly linked to treatment-related toxicity. Additionally, some known risk factors for renal injury were reestablished and specific situations like GVHD were emphasized to have a role in the pathogenesis of acute renal injury. An important paucity in previous investigations was that the majority of them were conducted on heterogeneous patient groups with the exception of a few researches which really had paid attention to form uniform patient populations.
The goal of the present study was to determine the incidence and risk factors of ARF and to investigate whether there exists a relationship between the renal injury indicators and the quantity of transplanted hematopoietic stem cells in a uniform patient population after allogeneic myeloablative HSCT.
METHODS
Patient selection and initial evaluation
This is a single-center prospective clinical study which started in March 2007 at the Bone Marrow Transplantation Unit of Erciyes University in Kayseri, after the approval of the local ethics committee. The time period for recruiting patients was ended in March 2008. To determine the baseline clinical and laboratory data, all eligible patients who signed the informed consent form were closely monitored prospectively in terms of their renal functions during the first 100 days after HSCT.
The study is specifically focused on the myeloablative allogeneic HSCT because of the established high risk for ARF. For the purpose of constituting a uniform patient population, patients who did not have HLA full-matched related donors (four patients) in addition to those who did not have entirely the same conditioning regimen (three patients) were excluded. Hence, a total of 39 myeloablative allogeneic HSCT patients were included after excluding 26 autologous and/or non-myeloablative HSCT patients.
To have a detailed evaluation, patients' medical histories, physical examinations, baseline laboratory testings, chest X-rays, electrocardiograms, and echocardiographies were taken and creatinine clearances were determined from urine samples collected for 24 hours. Adequate cardiac, liver, pulmonary, and renal functions were prerequisites to be accepted for HSCT. Patients were hospitalized to receive the conditioning chemotherapy regimen whenever they completed this initial evaluation.
Conditioning and HSCT procedure
All of the patients received the same conditioning regimen. None of them was treated with total body irradiation (TBI). Busulphan and cyclophosphamide were administered through a central venous catheter over 1–2 hours with doses of 3.2 and 50 mg/kg/body weight, 7 and 3 days before the infusion of the stem cells, respectively.
Cyclosporine and prednisolone were used together in all patients for the prophylaxis against GVHD. Prophylaxis was administered against Pneumocystis carinii, fungal infections, and cytomegalovirus with trimethoprim-sulfametaxazole, fluconazole, and acyclovir, respectively.
The sources of donor hematopoietic cells were granulocyte colony-stimulating factor-stimulated peripheral blood hematopoietic cells in all patients. The amount of collected CD34+ hematopoietic stem cells was determined by enumeration using a single-platform assay, ProCOUNTTM (Becton Dickinson Biosciences, San Jose, CA, USA) in which the absolute number of CD34+ cells is directly derived from a single flow cytometric measurement. The above-mentioned technique for CD34+ cell enumeration is described in detail elsewhere.Citation10 Finally, the collected donor hematopoietic stem cells were infused on day “zero” and all consequent days were numbered starting from that day on.
Patient monitoring
Patients were regularly assessed during their hospital stay for complications, predisposing to acute renal injury, including hypotension (defined as systolic blood pressure <90 mmHg), sepsis (defined by positive blood cultures indicating bacteremia or fungemia), and organ toxicities. All patients were monitored for signs and symptoms of GVHD and hepatic veno-occlusive disease. Total serum bilirubin, cyclosporine levels, serum albumin, blood urea nitrogen, and serum creatinine were measured on days 7, 14, 21, 30, 60, and 100. To measure cyclosporine blood concentration, a sample of venous blood was drawn 2 hours after the cyclosporine dose was given. All medications were recorded and analyzed separately in terms of their risk for nephrotoxicity. Hepatic veno-occlusive disease was defined as finding of at least twofold increases in total bilirubin levels and transaminases with ascites.Citation11
ARF was defined as a twofold rise in serum creatinine concentration of baseline value or a >50% decrease in creatinine clearance. Patients who developed acute kidney injury (AKI) were divided into three categories as given in .
TABLE 1. Classification of ARF
Urine microprotein/creatinine ratio was assessed in each patient to evaluate proteinuria. Urine samples for this process were provided from urine collected for 24 hours for creatinine clearance measurement.
Statistical analysis
SPSS 11.0 software was used for the statistical analysis and the Kolmogorov–Smirnov test to determine the normality of the distributions of variables. Continuous variables with normal distribution are presented as mean ± SD. Median value is used where normal distribution is absent. Statistical analysis for the parametric variables was performed using the Student's t-test between the two groups. The Mann–Whitney U-test was used to compare non-parametric variables between the two groups. Qualitative variables were given as percentages and the correlation between categorical variables was investigated using the chi-square test. A p-value of <0.05 was considered to be significant.
RESULTS
Baseline characteristics of 39 allogeneic HSCT patients are summarized in . The mean age of the 39 patients was 30.97 ± 12.33 years; 24 of the 39 patients were male. Acute myeloid leukemia and acute lymphoblastic leukemia were the most frequent primary hematologic diseases. Baseline mean serum creatinine level and baseline mean creatinine clearance of the 39 patients were 0.71 ± 0.19 mg/dL and 133.03 ± 44.54 mL/min/1.73 m2, respectively.
TABLE 2. Baseline characteristics of the allogeneic HSCT patients (n = 39)
None of the patients developed symptomatic hypovolemia due to vomiting and diarrhea because of extensive toxicity of myeloablative regimens during the 100 days of the study period.
ARF developed in 20 (51.3%) of the 39 patients. Out of the 20 patients, 11 (55.0%) and 9 (45.0%) developed stage I and stage II ARF, respectively. On the contrary, no patient developed stage III ARF. ARF developed in 12 (60.0%) patients within the first 2 weeks and in 8 (40.0%) patients within 2–4 weeks. No patient developed hepatic veno-occlusive disease, thrombotic microangiopathy, or TLS.
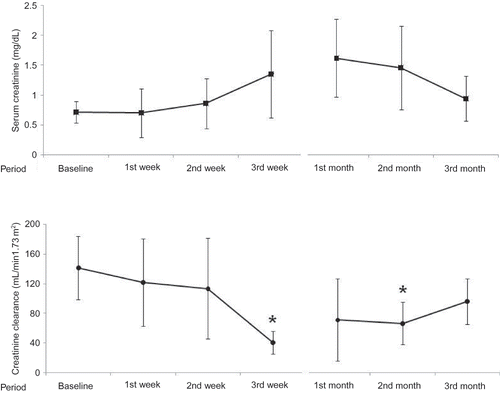
Comparison of demographic, clinical, and biochemical parameters in patients with and without ARF is summarized in . Serum creatinine levels at the second and third weeks and first and second months were significantly higher in patients with ARF than in controls (p: 0.022, 0.003, 0.001, and 0.001, respectively) (). The values of creatinine clearance at the third week and first and second months were meaningfully lower in ARF group compared to the control group (p: 0.003, 0.027, and 0.003, respectively) ().
TABLE 3. Demographic, clinical, and biochemical parameters in patients with and without ARF
FIGURE 1. Trends of serum creatinine levels and creatinine clearance after myeloblative HSCT. *p < 0.05 when compared to baseline values. 254 × 190 mm (96 × 96 DPI).
Body surface area was higher in ARF group than in controls (p: 0.046). Basal hemoglobin level was higher in ARF group when compared to controls (p: 0.007). On the contrary, there was no significant difference between the two groups in terms of age, gender, primary hematologic disease, engraftment period, presence of GVHD, clinical response after allogeneic HSCT, presence of sepsis, presence of coexistent disease, use of amphotericin-B and vancomycin, CD34+ cell count per kilogram of body weight, cyclosporine level, serum albumin level, serum hemoglobin level, white blood cell (WBC) count, platelet count, amount of proteinuria, and 24-hour urine volume (p > 0.05).
Demographic, clinical, and biochemical parameters in patients with stage I ARF or stage II ARF are given in . When patients with ARF were evaluated with regard to the clinical response to allogeneic HSCT, all patients with stage II ARF and four of the nine patients with stage I ARF were in remission. Two patients with stage I ARF gave no response to treatment whereas three of them were in partial remission.
TABLE 4. Demographic, clinical, and biochemical parameters in patients with stage I or stage II ARF
Values of creatinine clearance at the third week and second month were significantly lower in patients with stage II ARF than in patients with stage I ( p: 0.001 and 0.026, respectively). But there was no significant difference between the patients with stage I and stage II ARF for the values of creatinine clearance at basal, first week, second week, first month, and third month (p > 0.05).
Hemoglobin level at the second week was lower in patients with stage II ARF than in those with stage I (p: 0.043) whereas it was higher in patients with stage II compared with those with stage I at the third month (p: 0.018). However, there was no difference between two groups in terms of hemoglobin levels at basal, first week, third week, first month, and second month (p > 0.05). Cyclosporine level at the third week was higher in patients with stage II than in those with stage I (p: 0.019). However, there was no significant difference with regard to cyclosporine levels at basal, first week, second week, first, second, and third months (p > 0.05). There was no significant difference between the two groups in terms of age, gender, engraftment period, primary hematologic disease, body surface area, development time of ARF, presence of GVHD, presence of sepsis, presence of coexistence disease, use of amphotericin-B and vancomycin, baseline serum creatinine level, CD34+ cell count, serum albumin level, WBC count, platelet count, amount of proteinuria, 24-hour urine volume, and duration of ARF (p > 0.05).
Three patients died during the first 100 days after HSCT. One of them died on the 14th day because of neutropenic fever, gastroenteritis, and sepsis. The second patient died on the 72nd day because of pulmonary infection and pulmonary failure. Both developed ARF 7 days before death. The third patient died on the 74th day because of recurrence of primary hematological malignancy without any findings of ARF.
DISCUSSION
HSCT has become an effective treatment for some advanced malignant and non-malignant hematological malignancies. Despite many advances in immunosuppressive regimens and patient care, ARF remains to be a frequent complication after HSCT and leads to remarkable morbidity and mortality.Citation12 On the contrary, ARF is emerging as a public health problem worldwide.Citation13 Despite technical improvements in dialysis and intensive care, mortality and morbidity among patients with severe ARF remain high. Several therapies, including infusion of the stem cells that can accelerate renal recovery, have been attempted in experimental models of ARF.Citation14,Citation15 Hematopoietic stem cells are recognized to have an ability to differentiate into multiple lineages of hematopoietic and non-hematopoietic tissues such as cardiac myocytes, hepatocytes, gastrointestinal epithelial cells, and vascular endothelial cells.Citation16–20 Furthermore, it has been reported that adult bone marrow-derived mesenchymal stem cells or hematopoietic stem cells have a role in the recovery of the injured kidney tissues.Citation5,Citation8,Citation21 Mechanisms underlying their healing effect are still controversial.
It was initially suggested by Zager et al. that ARF after HSCT is a very frequent and devastating complication.Citation1 Its incidence was first reported to be 53% by Zager et al., and afterward subsequent studies confirmed this unusually high incidence of ARF after HSCT.Citation3,Citation4,Citation22,Citation23
The incidence of ARF was found to be 51.3% in our cohort. The first 4 weeks after HSCT seemed to have the highest risk for developing ARF in our patients in accordance with the previous reports.Citation1,Citation3,Citation24 The overall mortality, after the first 100 days after HSCT, was 7.7%, whereas the mortality rate in patients who developed ARF was 10% in the present study. When compared to previous studies, the lower mortality rate in our study was probably related to the severity of ARF. As mentioned above, none of our patients developed dialysis requiring ARF. According to a metaanalysis, the relative risk of death after ARF in myeloablative allogeneic HSCT was greater than twofold higher than those without ARF.Citation24 However, it has been suggested that the degree of ARF after HSCT is particularly correlated with mortality.Citation1
It has been clarified recently that the characteristics of ARF vary among the three types of transplantations.Citation12 Myeloablative allogeneic HSCT carries the highest risk for ARF,Citation1,Citation4,Citation12,Citation22 when compared to non-myeloablative allogeneicCitation24,Citation25 and myeloablative autologous transplantations.Citation1,Citation26,Citation27 Myeloablation requires high-dose chemotherapy with or without TBI that may cause severe vomiting, diarrhea, and renal injury. The risk for GVHD increases in myeloablative allogeneic transplantation, and GVHD may contribute to nephrotoxicity.Citation28,Citation29 Our myeloablative regimen did not include TBI, and this might have had a decreasing effect on renal injury in our patients. In addition, we did not find any significant correlation between GVHD and ARF development. Although the latter finding is in accordance with most of the previous studies,Citation1,Citation3,Citation25 further research is needed to clarify whether there is a link between GVHD and ARF.
Patients with larger body surface area were found to be vulnerable to ARF. Although we assumed that ARF may be associated with insufficient amount of infused stem cells, we found no correlation between the number of infused stem cells per body weight and ARF development.
Calcineurin toxicity and TLS are also well-known causes for ARF. However, we did not find any correlation between blood cyclosporine levels and development of ARF. However, blood cyclosporine levels were significantly higher in patients with stage II ARF compared with patients with stage I ARF. None of our patients developed TLS in accordance with the finding that TLS in this population is reported to be quite low.Citation2 Furthermore, known risk factors such as use of amphotericin-B and sepsis did not seem to have a direct relationship with ARF development in our patients. We conclude that high incidence of ARF in this special group of patients cannot be linked to a single cause. Rather a combination of multiple risk factors seems to be responsible for developing ARF.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Zager RA, O'Quigley J, Zager BK, Acute renal failure following bone marrow transplantation: A retrospective study of 272 patients. Am J Kidney Dis. 1989;13(3):210–216.
- Zager RA. Acute renal failure in the setting of bone marrow transplantation. Kidney Int. 1994;46:1443–1458.
- Gruss E, Bernis C, Tomas JF, Acute renal failure in patients following bone marrow transplantation: Prevalence, risk factors and outcome. Am J Nephrol. 1995;15(6):473–479.
- Parikh CR, McSweeney PA, Korular D, Renal dysfunction in allogeneic hematopoietic cell transplantation. Kidney Int. 2002;62(2):566–573.
- Kale S, Karihaloo A, Clark PR, Kashgarian M, Krause DS, Cantley LG. Bone marrow stem cells contribute to repair of the ischemically injured renal tubule. J Clin Invest. 2003;112(1):42–49.
- Duffield JS, Park KM, Hsiao LL, Restoration of tubular epithelial cells during repair of the post-ischemic kidney occurs independently of bone marrow-derived stem cells. J Clin Invest. 2005;115:1743–1755.
- Toegel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol. 2005;289:F31–F42.
- Lin F, Cordes K, Li L, Hematopoietic stem cells contribute to the regeneration of renal tubules after renal ischemia-reperfusion injury in mice. J Am Soc Nephrol. 2003;14(5):1188–1199.
- Imberti B, Morigi M, Tomasoni S, Insulin-like growth factor-1 sustains stem cell-mediated renal repair. J Am Soc Nephrol. 2007;18:2921–2928.
- Sutherland DR, Anderson L, Keeney M, Nayar R, Chin-Yee I. The ISHAGE guidelines for CD34+ cell determination by flow cytometry: International Society of Hematotherapy and Graft Engineering. J Hematother. 1996;5(3):213–226.
- Deleve LD, Shulman HM, McDonald GB. Toxic injury to hepatic sinusoids: Sinusoidal obstruction syndrome (venocclusive disease). Semin Liver Dis. 2002;22:27–41.
- Parikh CR, Coca SG. Acute renal failure in hematopoietic cell transplantation. Kidney Int. 2006;69:430–435.
- Mehta RL, Kellum JA, Shah SV, Acute kidney injury network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31.
- Star RA. Treatment of acute renal failure. Kidney Int. 1998;54:1817–1831.
- Kelly KJ, Molitoris BA. Acute renal failure in the new millennium: Time to consider combination therapy. Semin Nephrol. 2000;20:4–19.
- Lagasse E, Connors H, Al-Dhalimy M, Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat Med. 2000;6:1229–1234.
- Orlic D, Kajstura J, Chimenti S, Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705.
- Okamoto R, Yajima T, Yamazaki M, Damaged epithelia regenerated by bone marrow-derived cells in the human gastrointestinal tract. Nat Med. 2002;8:1011–1017.
- Shimizu K, Sugiyama S, Aikawa M, Host bone-marrow cells are a source of donor intimal smooth-muscle-like cells in murine aortic transplant arteriopathy. Nat Med. 2001;7:738–741.
- Takahashi T, Kalka C, Masuda H, Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434–438.
- Poulsom R, Forbes SJ, Hodivala-Dilke K, Bone marrow contributes to renal parenchymal turnover and regeneration. J Pathol. 2001;195:229–235.
- Nash RA, Antin JH, Karanes C, Phase 3 study comparing methotrexate and tacrolimus with methotrexate and cyclosporine for prophylaxis of acute graft-versus-host disease after marrow transplantation from unrelated donors. Blood. 2000;96:2062–2068.
- Parikh CR, Schrier RW, Storer B, Comparison of ARF after myeloablative and nonmyeloablative hematopoietic cell transplantation. Am J Kidney Dis. 2005;45:502–509.
- Parikh CR, McSweeney PA, Schrier RW. Acute renal failure independently predicts mortality after myeloablative allogeneic hematopoietic cell transplant. Kidney Int. 2005;67:1999–2005.
- Parikh CR, Sandmaier BM, Storb RF, Acute renal failure after nonmyeloablative hematopoietic cell transplantation. J Am Soc Nephrol. 2004;15:1868–1876.
- Merouani A, Shpall EJ, Jones RB, Archer PG, Schrier RW. Renal function in high dose chemotherapy and autologous hematopoietic cell support treatment for breast cancer. Kidney Int. 1996;50:1026–1031.
- Fadia A, Casserly LF, Sanchorawala V, Incidence and outcome of acute renal failure complicating autologous stem cell transplantation for AL amyloidosis. Kidney Int. 2003;63:1868–1873.
- Rao PS. Nephrotic syndrome in patients with peripheral blood stem cell transplant. Am J Kidney Dis. 2005;45:780–785
- Panoskaltsis-Mortari A, Price A, Hermanson JR, In vivo imaging of graft-versus-host-disease in mice. Blood. 2004;103:3590–3598.
