Abstract
Objectives: To conduct a systematic review of the literature to summarize the best available evidence regarding the mortality and morbidity associated with differing dosing regimens of continuous renal replacement therapy (CRRT) for patients with acute renal failure (ARF) in an intensive care unit setting. Patients and Methods: We searched for randomized controlled trials in electronic databases from January 1990 through November 2009. Eligible trials compared two or more dosing regimens of CRRT in patients with ARF. Two reviewers working independently determined trial eligibility and extracted descriptive, methodological, and outcome data. Random-effects meta-analysis was used to assess relative risks (RR) and weighted mean difference. The I2-statistic was used to assess heterogeneity of treatment effect across trials. Results: Seven trials were eligible for meta-analysis. We found no reduction in mortality in patients who received higher doses of CRRT (RR 0.88, 95% CI 0.75–1.03, I2 = 74%). There was no difference in the requirement of renal replacement therapy at the conclusion of the study period (RR 1.12, 95% CI 0.86–1.46, I2 = 3%). The overall quality of evidence was downgraded because of imprecision and heterogeneity. Conclusion: Increased dosing of CRRT is not associated with a decrease in mortality of patients with ARF in an intensive care unit setting.
INTRODUCTION
Acute renal failure (ARF) affects up to 5% of patients with critical illness admitted to the intensive care unit (ICU).Citation1 When factored independent from comorbidities, ARF increases the risk of death by fourfold.Citation2 Over two decades ago, continuous renal replacement therapy (CRRT) was introduced and expanded the dialysis options for critically ill patients from traditional, intermittent hemodialysis (IHD) techniques. Despite new renal replacement techniques, the mortality associated with ARF has not changed over the past four decades.Citation3 There are wide practice variations in CRRT that is delivered to the critically ill patient with no consensus on the modality, timing of initiation, frequency, dosing, or duration.Citation4 Additionally, other factors such as fluid resuscitation, training of caregivers, and the performance standards of CRRT apparatus vary considerably.Citation1,Citation5,Citation6
Clinicians who initiate CRRT for ARF are often confronted with a dilemma regarding the optimal prescribed CRRT dosage. Early studies suggested a significant reduction in mortality with escalated dose of CRRT,Citation7,Citation8 whereas other studies did not reach the same conclusion.Citation9–12 Further confounding the decision-making process is a trial demonstrating no difference in mortality with increased intensity of renal replacement therapy (RRT) using multiple RRT modalities.Citation11 We conducted a systematic review of the literature to summarize the best available evidence regarding possible mortality and morbidity associated with differing prescribed dosing regimens of CRRT.
MATERIALS AND METHODS
The report of this protocol-driven systematic review is in adherence with the Quality of Reporting of Meta-analyses standards (QUOROM) for reporting meta-analyses of randomized controlled trials (RCT).Citation13 Whenever possible, we used the nomenclature and definitions published by the Acute Dialysis Quality Initiative.
Eligibility criteria
Eligible studies were RCT that enrolled adult patients in a critical care setting with ARF and compared different dosing regimens of venous to venous CRRT. To be eligible, studies needed to measure the outcomes of interest: death, ICU length of stay, hospital length of stay, duration of RRT, need for RRT at the conclusion of the study period, improvements in fluid balance, or the need for vasopressor support. We excluded review articles, articles without original data, and observational studies. Because continuous renal replacement techniques using an arterial access are not comparable to techniques utilizing venous to venous access, we excluded studies of patients with arterial access for CRRT.
Study identification
An expert reference librarian (PJE) designed and conducted the electronic search strategy after input from study investigators with expertise in conducting systematic reviews. We searched electronic databases from January 1990 through November 2009 (MEDLINE, EMBASE, Cochrane CENTRAL, Web of Science, Scopus; Regional Medical databases: KoreanMed, Scielo, LILACs, Imbiomed, Eastern Mediterranean Index, IndMed, ExtraMed). The strategy utilized a combination of controlled subject headings where available and text words to describe the concepts of interest. In MEDLINE, regional MEDLINES, EMBASE, and CENTRAL, the terms included renal dialysis, renal replacement therapy, hemodiafiltration, hemofiltration, kidney failure, critical illness, critically ill, and acute/therapy in conjunction with intensive care units. EMBASE included specific terms for CRRT. Specific outcomes of interest were mortality, length of stay, treatment outcomes; we also searched for controlled trials, meta-analyses, comparative studies, or systematic reviews. Text words were employed with appropriate synonyms and abbreviations in the other keyword-based databases. We also sought references from experts, bibliographies of included trials, and the ISI Science Citation Index for publications that cited included studies.
Data collection
Two reviewers (ETC and BPG) working independently and blindly using a standardized form extracted descriptive, methodological, and outcome data from all eligible studies. Inter-reviewer agreement was measured using the kappa statistic. We attempted to contact authors of all included studies by e-mail to obtain missing data.
Meta-analyses
From each trial, we pooled the relative risks (RR) for dichotomous outcomes and the weighted mean difference (WMD) for continuous outcomes. Anticipating significant heterogeneity in CRRT methods, settings, and patients, we used the DerSimonian and Laird random-effects model.Citation14 We estimated the 95% confidence intervals for each outcome and calculated the I2-statistic which represents the proportion of variability across trials that is not attributable to chance.Citation15 Statistical analysis was conducted using Comprehensive Meta-Analysis, Version 2 (Biostat Inc., 2005, Englewood, NJ, USA).
Sensitivity, subgroup, and publication bias analyses
We planned to repeat analysis using the fixed effect model to determine whether the choice of statistical model affects study conclusions. To explain possible heterogeneity, we planned to conduct subgroup analyses based on patients' gender, age (<65 vs. ≥65 years), the presence of diabetes, sepsis, chronic kidney disease, and type of ICU admission (medical, surgical, cardiac surgery). We tested whether the methodological quality of the study, mainly driven by allocation concealment as blinding is not feasible, would affect study conclusions. Treatment effect–subgroup interactions were assessed by the analysis of variance method (ANOVA) with two-tailed alpha set at 0.05. Meta-regression was used to test the effect of the length of study follow-up and the severity of illness score on the effect size (log RR). We visually inspected funnel plots and conducted Egger's regression test to evaluate publication bias. In this regression model, precision is used to predict the standardized effect size; the size of the treatment effect is captured by the slope of the regression line and bias is captured by the intercept.Citation16
RESULTS
Study identification
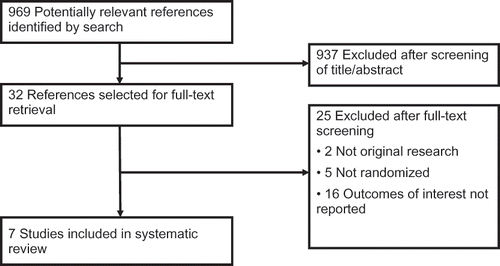
Our search and selection procedure is depicted in . We found seven eligible trials that compared different prescribed doses of venous to venous CRRT to critically ill patients with ARF (3545 participants, mean sample size of 506).Citation7–12,Citation17 The mean age of patients was 64 years. describes the patient characteristics at the time of study randomization. On admission to ICU, 49% of the patients had sepsis; 33% had history of chronic kidney disease; and 31% were surgical or trauma patients. The median study period for mortality and the need for continuing RRT were 60 days and 71 days, respectively. summarizes the characteristics of the included studies. Authors of six of these studies responded to our request for missing information.Citation8–11,Citation17 We excluded one small trial as the dosage of CRRT and patient demographics were not reported.Citation18 Repeated attempts to contact the primary author for further data were unsuccessful.
TABLE 1. Baseline characteristics of patients at the time of randomization
TABLE 2. Study characteristics
Methodological quality
summarizes the methodological quality of the included studies. Reviewers had adequate chance-adjusted agreement in judging study quality (k = 1.00). Overall, the studies were unblinded, and allocation of study participants was not concealed. There were no patients lost to follow-up in three of the trials.Citation7–9 Funding sources were nonprofit, nonprofit and industry, or not reported.
TABLE 3. Study quality
Meta-analysis
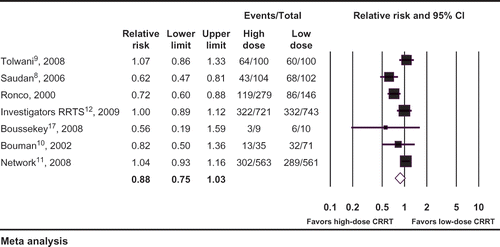
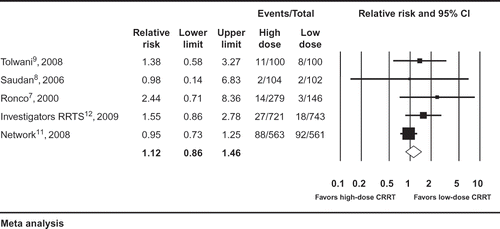
Pooling results from the seven trials that compared different doses of CRRT demonstrated no reduction in mortality in patients who received higher doses of CRRT (seven studies: RR 0.88, 95% CI 0.75–1.03, I2 = 74%, ). There was no difference for the requirement of RRT at the conclusion of the study period (five studies: RR 1.12, 95% CI 0.86–1.46, I2 = 3%, ), ICU length of stay (five studies: WMD –0.08 days, 95% CI –1.13 to 0.98, I2 = 31%,), or hospital length of stay (four studies: WMD 0.65 days, 95% CI –0.81 to 2.10, I2 = 0%). The outcome of vasopressor requirements was reported in only one studyCitation17 and showed a decrease in the mean norepinephrine dosing >75% at 24 hours with higher prescribed dosage of CRRT (p = 0.004).
Sensitivity, subgroup, and publication bias analyses
Meta-regression did not demonstrate an association between the length of study follow-up and the severity of illness score on the effect size although this analysis was clearly underpowered. Study quality did not affect the effect size (high quality vs. low quality; p-value for the test of interaction for the outcomes of death and requirement of long-term RRT was 0.13 and 0.20, respectively). Data were insufficient to conduct several other planned subgroup analyses. There was no evidence for publication bias as demonstrated by visual inspection of funnel plots or by Egger's test (p-value for the outcomes of death and requirement of long-term RRT was 0.17 and 0.21, respectively). The use of fixed effect model did not change study conclusions. The exclusion of the RENAL studyCitation12 (as it had the most weight in meta-analysis) or the ARF Trial Network studyCitation11 (in which integrated HD strategies were used) or Bousskey et al.Citation17 (in which the population seemed older and all had septic shock) did not change study conclusions (RRs respectively: 0.84, 95% CI 0.67–1.04; 0.83, 95% CI 0.68–1.02; and 0.89, 95% CI 0.75–1.04). Considering the date of study publication as a source of heterogeneity shows that earlier studiesCitation7,Citation8,Citation10 published between 2000 and 2006 did in fact show a reduction in mortality (RR 0.70, 95% CI 0.60–0.81); however, these studies were quite small. Larger studies with markedly more events and power were contradictory and drove the pooled estimate clearly toward a no effect on mortality.
DISCUSSION
Early evidence suggested that higher dosages of CRRT may improve patient outcomes. Studies conducted on animals that were given systemic endotoxins followed by CRRT suggested improved hemodynamics and cytokine removal with high-volume hemofiltration.Citation19–21 A large retrospective study found that higher dosages of CRRT lead to decreased mortality in a subset of patients.Citation22 Another study showed improved acid–base balance and increased uremic clearance with higher dosage of CRRT.Citation23 Although high-efficiency blood purification may be achieved with CRRT or IHD, the optimal balance of efficiency, safety, and improved patient outcomes continues to be investigated.
We conducted a systematic review that demonstrated no statistical difference toward decreased mortality in patients receiving higher dosage of CRRT as treatment for ARF in an ICU setting. We did not find a difference in ICU or hospital length of stay, or the need for RRT at the end of study period between lower or higher dosages of CRRT. The quality of evidence generated by these randomized trials was downgraded because of imprecision (CIs that include both harm and benefit) and the significant heterogeneity of treatment effect on the outcome of mortality across trials that could not be explained by subgroup interactions or meta-regression.
Although in a typical clinical setting patients with ARF may receive both intermittent and continuous forms of RRT, we included studies of patients in an ICU setting with ARF in whom treatment with CRRT is started and a prescribed dosage is required, also a common clinical scenario. As we conducted a systematic review of the literature to summarize the best available evidence regarding the mortality and morbidity associated with differing dosing regimens of CRRT for patients with ARF in an ICU setting, we did not include studies of IHD dosing. Of the studies we included, there was insufficient reporting for analysis of the frequency, dose, or duration of IHD that patients may have received after CRRT was discontinued. Patients enrolled in the Intensity of Renal Support in Critically Ill Patients with Acute Kidney InjuryCitation11 and the Randomized Control Trial of Normal versus Augmented Level of Renal Replacement Therapy Citation12 comprised about 73% of the patients in our systematic review, and the results of these studies heavily weighted our findings. However, the exclusion of these trials from analysis does not change the conclusions of this review regarding any outcome.
Strengths and limitations of this review
The strengths of this review stem from the comprehensive search strategy and the bias protection measures taken by reviewers, such as reviewing in duplicate and author contact. We conducted several a priori exploratory analyses looking for causes of heterogeneity and publication bias.
Although blinding of patients and caregivers may not be feasible in CRRT studies, allocation concealment and blinding of data collectors and outcome assessors are possible and desirable. The RCT included in this review had inconsistent allocation concealment and non-blinded outcome and data collection. Due to these methodological limitations, as well as the statistical imprecision and heterogeneity, the quality of evidence presented in this review is considered of lesser quality (i.e., at higher risk of bias). In addition, although we found no evidence of publication bias, the methods used can miss the presence of such bias when the number of included studies is small.Citation24 We were unable to test certain patient characteristics that would have been very useful clinically and may have explained heterogeneity. These characteristics are difficult to ascertain via subgroup analysis in a summary-data meta-analysis such as this one because of lack of power, ecological bias, and sparse data and are best evaluated in a large RCT or in a patient-level meta-analysis.
CONCLUSIONS
Evidence of moderate to low quality demonstrated no change in mortality with escalated dosing of CRRT for patients with ARF in an ICU setting. The need for continuing RRT, or patient ICU and hospital length of stay were also unaffected by the dose of CRRT.
Acknowledgments
This research was financed solely with internal funding by the Mayo Clinic, Rochester, Minnesota.
REFERENCES
- Uchino S, Kellum JA, Bellomo R, Acute renal failure in critically ill patients: A multinational, multicenter study [see comment]. JAMA. 2005;294:813–818.
- Metnitz PGH, Krenn CG, Steltzer H, Effect of acute renal failure requiring renal replacement therapy on outcome in critically ill patients [see comment]. Crit Care Med. 2002;30:2051–2058.
- Ympa YP, Sakr Y, Reinhart K, Vincent J-L. Has mortality from acute renal failure decreased? A systematic review of the literature. Am J Med. 2005;118:827–832.
- Overberger P, Pesacreta M, Palevsky PM, Network VNARFT. Management of renal replacement therapy in acute kidney injury: A survey of practitioner prescribing practices. Clin J Am Soc Nephrol. 2007;2:623–630.
- National Heart LaBIARDSCTN, Wiedemann HP, Wheeler AP, Comparison of two fluid-management strategies in acute lung injury [see comment]. New Engl J Med. 2006;354:2564–2575.
- Ricci Z, Ronco C, D'Amico G, Practice patterns in the management of acute renal failure in the critically ill patient: An international survey. Nephro Dial Transplant. 2006;21:690–696.
- Ronco C, Bellomo R, Homel P, Effects of different doses in continuous veno-venous hemofiltration on outcomes of acute renal failure: A prospective randomised trial [see comment]. Lancet. 2000;356:26–30.
- Saudan P, Niederberger M, De Seigneux S, Adding a dialysis dose to continuous hemofiltration increases survival in patients with acute renal failure [see comment]. Kidney Int. 2006;70:1312–1317.
- Tolwani AJ, Campbell RC, Stofan BS, Lai KR, Oster RA, Wille KM. Standard versus high-dose CVVHDF for ICU-related acute renal failure [see comment]. J Am Soc Nephrol. 2008;19:1233–1238.
- Bouman CS, Oudemans-Van Straaten HM, Tijssen JG, Zandstra DF, Kesecioglu J. Effects of early high-volume continuous venovenous hemofiltration on survival and recovery of renal function in intensive care patients with acute renal failure: A prospective, randomized trial [see comment]. Crit Care Med. 2002;30:2205–2211.
- Network VNARFT, Palevsky PM, Zhang JH, Intensity of renal support in critically ill patients with acute kidney injury [see comment]. New Engl J Med. 2008;359:7–20.
- Investigators RRTS, Bellomo R, Cass A, Intensity of continuous renal-replacement therapy in critically ill patients [see comment]. New Engl J Med. 2009;361:1627–1638.
- Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6:e1000097.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188.
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560.
- Borenstein M. Introduction to Meta-Analysis. 1st ed. Chichester: John Wiley & Sons; 2009.
- Boussekey N, Chiche A, Faure K, A pilot randomized study comparing high and low volume hemofiltration on vasopressor use in septic shock. Intensive Care Med. 2008;34:1–8.
- Daud KM, Leong GB, Visvanathan R. Acute dialytic support for the critically ill: Continuous venovenous hemodialysis versus continuous venovenous hemofiltration. Int Med J. 2006;13:37–42.
- Bellomo R, Kellum JA, Gandhi CR, Pinsky MR, Ondulik B. The effect of intensive plasma water exchange by hemofiltration on hemodynamics and soluble mediators in canine endotoxemia. Am J Respir Crit Care Med. 2000;161:1429–1436.
- Gomez A, Wang R, Unruh H, Hemofiltration reverses left ventricular dysfunction during sepsis in dogs. Anesthesiology. 1990;73:671–685.
- Grootendorst AF, van Bommel EF, van der Hoven B, van Leengoed LA, van Osta AL. High volume hemofiltration improves right ventricular function in endotoxin-induced shock in the pig. Intensive Care Med. 1992;18:235–240.
- Paganini EP, Kanagasundaram NS, Larive B, Greene T. Prescription of adequate renal replacement in critically ill patients. Blood Purif. 2001;19:238–244.
- Brause M, Neumann A, Schumacher T, Grabensee B, Heering P. Effect of filtration volume of continuous venovenous hemofiltration in the treatment of patients with acute renal failure in intensive care units. Crit Care Med. 2003;31:841–846.
- Lau J, Ioannidis JPA, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. BMJ. 2006;333:597–600.