Abstract
Objective: Epidemiological and experimental studies indicate that kidney disease is associated with increased oxidative stress. Our aim was to determine whether paraoxonase 1 (PON1) activity is altered in patients with moderately decreased glomerular filtration rates (GFRs) compared to healthy controls. Material and methods: Forty-eight patients showing relatively low GFRs upon renal scintigraphy with 99mTc-DTPA were compared to 40 age-matched healthy subjects. Serum PON1 activity was measured spectrophotometrically. Lipid hydroperoxide levels were measured via iodometric assay. Results: The mean ages of the patient and control groups were 32.09 ± 6.10 (range 23–50) and 31.30 ± 5.30 (range 20–46) years, respectively. Serum PON1 (p= 0.949) and high-density lipoprotein (p= 0.473) levels did not differ between groups. Significant differences were detected between groups in terms of mean triglyceride (p= 0.009), very-low-density lipoprotein (p = 0.010), lipid hydroperoxide (p = 0.026), urea (p = 0.012), and creatinine (p = 0.001) levels, whereas total cholesterol (p = 0.520) and low-density lipoprotein (p = 0.161) were similar between groups. Mean GFR was significantly lower in the low GFR group compared to the control (p = 0.000). Conclusion: Our results indicate that PON1 activity and high-density lipoprotein levels may not be determining factors in premature vascular aging in patients with moderately decreased GFRs. Instead, some other undetermined factor(s) may be involved in modulating enzymatic activity.
INTRODUCTION
Numerous anatomical and functional changes in the kidney lead to decreased renal blood flow and subsequent decreases in glomerular filtration rate (GFR). This condition, known as impaired renal autoregulation, may lead to an earlier death in patients with chronic kidney disease (CKD).Citation1,Citation2 For example, data from the third National Health and Nutrition Examination Survey (NHANES III) suggest that the prevalence of CKD among adults in the United States may be as high as 11%, constituting 19.2 million individuals.Citation3 Recently, several studies have demonstrated that low-density lipoprotein (LDL) is more susceptible to oxidation in CKD patients, suggesting that antioxidative mechanisms may be impaired in these individuals.Citation4,Citation5 Similarly, LDL oxidation appears to play a key role in atherosclerosis.Citation6 During the previous 15 years, a great deal of information regarding the basic physiology and pathology of reduced GFR has been reported. These include many disorders that effect vascular and renal systems such as atherosclerosis, diabetes mellitus, cardiac insufficiency, glomerulopathies, and aging. However, several controversial points remain, one of which being the relationship between the high-density lipoprotein (HDL)-dependent antioxidant enzyme, paraoxonase 1 (PON1), and decreased GFR.
PON1 protects LDL and HDL from lipid peroxidation and it is thought to function as the primary anti-atherosclerotic component of HDL. There are at least two enzymes present in HDL, PON1,Citation7–9 and platelet-activating factor acetylhydrolase, which have been shown to prevent the formation of oxidized LDL in vitro.Citation10 PON1 hydrolyzes oxidized lipids, inhibits cell-mediated LDL oxidation, and protects macrophages from oxidative stress by decreasing the uptake of oxidized LDL; furthermore, PON1 inhibits cholesterol biosynthesis in macrophages.Citation11
Previous studies showed that individuals with low PON1 activities are susceptible to atherosclerosis-related pathologies, such as diabetes mellitus, familial hypercholesterolemia, and renal disease.Citation12 Serum high LDL/lipid levels, low PON1 activity, low HDL levels, and a number of other major and minor factors cause to increase the risk of coronary artery disease,Citation13–15 which is the primary cause of death in approximately 50% of CKD patients.Citation16,Citation17
Lipid peroxidation leads to the generation of lipid hydroperoxides (LOOHs) from unsaturated phospholipids, glycolipids, and cholesterol. LOOHs mediate peroxidative reactions and are typically more persistent than any free radical precursor, making intermembrane translocation within a cell, between cells, or between lipoproteins and cells possible.Citation18 LOOHs can be used as an indicator of oxidative stress in cells and tissues, and increased levels of lipid peroxidation products have been observed in a variety of diseases in both humans and model systems.Citation19 Our objective was to elucidate the role of oxidative stress in patients with moderately decreased GFRs and to determine whether PON1 activity differs in these patients, as compared to healthy controls.
MATERIAL AND METHODS
Forty-eight patients (12 (25%) females) and 36 (75%) males) showing relatively low GFRs upon routine laboratory tests and renal scintigraphy with 99mTc-DTPA were enrolled, and 40 age-matched men were included as control. This study was approved by our institutional review board and was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki. All participants provided written informed consent. Patients and control subjects who had undergone renal surgery or developed a systemic disease, such as malignancy, diabetes mellitus, acute coronary disease, hypertension, renal disease, fever, or renal stones, were excluded. Patients who were taking anti-lipidemic drugs or steroids or who showed elevated liver enzyme levels were also excluded.
Blood sample collection
Serum samples were collected from patients and controls at 9:00 and 11:00 a.m. after an overnight fast. The samples were centrifuged within 2 h after withdrawal and stored at −80°C until analysis. Blood was analyzed only once.
Measurement of PON1
PON1 activity was assayed using paraoxon as a substrate, as previously described.Citation19 Briefly, PON1 activity was measured at 25°C by adding 50 μL of serum to 1 mL Tris–HCl buffer (100 mM at pH 8.0) containing 2 mM CaCl2 and 5.5 mM of paraoxon. Increased absorbance at 412 nm as a result of 4-nitrophenol formation was used as an indicator of PON1 activity. Enzymatic activity was calculated by using the molar extinction coefficient 17100 M−1 cm−1.
Lipid profile analysis
Serum levels of triglyceride (TG), total cholesterol (TC), HDL, LDL, and very-low-density lipoprotein (VLDL) were measured using commercially available assay kits (Abbott, Turkey) and an autoanalyzer (Aeroset, Abbott, Turkey).
Measurement of LOOH levels
Tri-iodide complex formation as a result of reaction between LOOH and iodine was evaluated via spectrophotometry at 365 nm. LOOH levels were calculated using the extinction coefficient of tri–iodide (ε = 2.46 × 104 M−1 cm−1).Citation20
Statistical analysis
Results are expressed as the mean ± SD for all continuous variables. Differences between patient and control groups were assessed using Student's t-test. The relationship between PON1 activities and LOOH level was evaluated using Spearman's correlation, and serum lipids were evaluated using Pearson's correlation test.
RESULTS
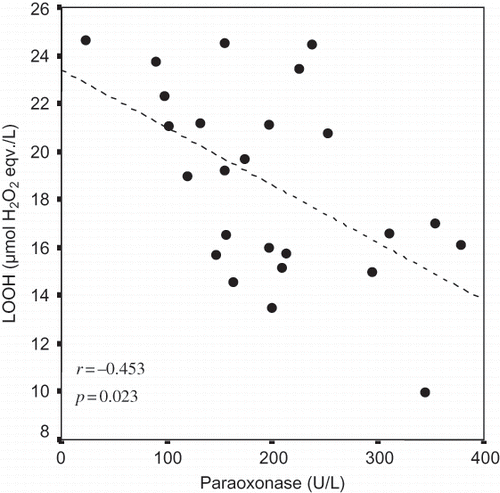
The mean ages of the low GFR and control groups were 32.09 ± 6.10 years (range 23–50 years) and 31.30 ± 5.30 years (range 20–46 years), respectively (p > 0.05). Serum PON1 activity (p = 0.949) and HDL (p = 0.473) levels did not differ between groups. Significant differences were detected between groups in terms of mean TG (p = 0.009), VLDL (p = 0.010), LOOH (p = 0.026), urea (p = 0.012), and creatinine (p = 0.001) levels, whereas TC (p = 0.520) and LDL (p = 0.161) were similar between groups (). Mean GFR was significantly lower in the low GFR group, compared to the control (p = 0.000). Furthermore, among all subjects, we observed a negative correlation between PON1 and LOOH activity (r = −0.453, p = 0.023) ().
TABLE 1. Serum PON1 activity and LOOH, HDL, mean serum lipid, urea, creatinine levels, mean GFR rate values in patient and control groups
DISCUSSION
The National Kidney Foundation Kidney Disease Outcomes Quality Initiative has recently adopted the use of GFR to stage CKD. According to this classification, stage 3 CKD is defined by a moderately decreased GFR of 30–59 mL/min/1.73 m2. The NHANES III data suggest that there are more than 8 million adults in the United States with an estimated GFR of less than 60 mL/min/1.73 m2.Citation21 In this study, we examined an adult sample showing stage 3 CKD or higher. However, we did not observe a significant difference in PON1 activity and HDL levels between the two groups. These results are consistent with those of Payson et al.,Citation22 who found no significant correlation between any biomarker of oxidative stress or inflammation and GFR. A potential explanation for the limited correlation of biomarkers of oxidative stress and inflammation with GFR may be that many of the solutes used as biomarkers undergo renal clearance primarily via renal tubular metabolism rather than glomerular filtration.Citation23–25 However, several studies have associated changes in GFR with biomarkers of inflammation, particularly in patients with advanced disease.Citation26–29 National Kidney Foundation reported that oxidative stress was associated with stage 3 CKD. These stage 3 CKD patients were at risk for progression of kidney disease and the development of end-stage renal disease (ESRD). Furthermore, these patients appeared to be at even greater risk for the development of cardiovascular disease and associated morbidity and mortality. Thus, most patients with stage 3 CKD will die of cardiovascular complications prior to developing ESRD.Citation21 Another study demonstrated that decreased paraoxonase activity and the subsequent loss of its antiatherogenic effects in renal failure may be a key factor in premature vascular aging.Citation30 In this study, mean serum TC and LDL levels were similar between the low GFR and control groups. Similarly, Vanholder et al.Citation23 and Schiavon et al.Citation31 found no significant difference between lipid profiles of patients and control subjects.
In this study, we detected a negative correlation between PON1 activity and LOOH levels in all subjects, which may reflect the fact that LOOH is a physiological substrate of PON1. Thus, as PON1 activity decreases, LOOH levels would be expected to increase. Elevated serum LOOH may also be dependent upon serum TG and VLDL, which were significantly higher in the low GFR group. These results suggest that the level of paraoxonase activity prevents premature vascular aging in low GFR patients under high oxidative stress.
Previous studies have demonstrated a close relationship between CKD and oxidative stress. Bolton et al.Citation32 examined 17 CKD patients (GFR < 50 mL/min) and demonstrated an increase in autoantibody production against oxidized LDL.Citation24 Mezzano et al.Citation33 examined 64 advanced CKD patients and observed increases in lipid peroxidation and advanced oxidation protein products (AOPP). Witko-Sarsat et al.Citation34 examined a cohort of 162 uremic patients and detected a strong relationship between AOPP accumulation and decreased GFR.Citation34 In contrast, we did not observe a relationship between increased oxidative stresses and lower GFR in this study.
In conclusion, our results indicate that HDL levels and PON1 activity may not be determining factors in the accelerated development of premature vascular aging in patients with moderately decreased GFRs. Instead, some other undetermined factor(s) may modulate enzyme activity. Further prospective studies with larger sample sizes are required to clarify this issue.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Mcclellan WM, Knight DF, Karp H, Brown WW. Early detection and treatment of renal disease in hospitalized diabetic and hypertensive patients: Important differences between practice and published guidelines. Am J Kidney Dis. 1997;29:368–375.
- Kausz AT, Khan SS, Abıchandanı R, Management of patients with chronic renal insufficiency in the northeastern United States. J Am Soc Nephrol. 2001;12:1501–1507.
- Coresh J, Astor BC, Green T, Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2003;41:1–12.
- Panzetta O, Cominacini L, Garbin U, Increased susceptibility of LDL to in vitro oxidation in patients on maintenance hemodialysis: Effects of fish oil and vitamin E administration. Clin Nephrol. 1995;44:303–309.
- Cristol JP, Dantoine T, Morena M, Protective effects of HDL against oxidative stress is impaired in hemodialysis patients. Nephrol Dial Transplant. 1997;12:A156.
- Witztum IL. The oxidation hypothesis of atherosclerosis. Lancet. 1994;344:793–795.
- Mackness MI, Arrol S, Durrington PN. Paraoxonase prevents accumulation of lipoperoxides in low-density lipoprotein. FEBS Lett. 1991;286:152–154.
- Shih DM, Xia YR, Wang XP, Combined serum paraoxonase knockout/apolipoprotein E knockout mice exhibit increased lipoprotein oxidation and atherosclerosis. J Biol Chem. 2000;275:17527–17535.
- Watson AD, Berliner JA, Hama SY, Protective effect of high density lipoprotein associated paraoxonase. Inhibition of the biological activity of minimally oxidized low density lipoprotein. J Clin Invest. 1995;96:2882–2891.
- Watson AD, Navab M, Hama SY, Effect of platelet activating factor-acetylhydrolase on the formation and action of minimally oxidized low density lipoprotein. J Clin Invest. 1995;95:774–782.
- Aviram M, Rosenblat M. Paraoxonases 1, 2, and 3, oxidative stress, and macrophage foam cell formation during atherosclerosis development. Free Radic Biol Med. 2004;37:1304–1316.
- Mackness M, Mackness B. Paraoxonase 1 and atherosclerosis: Is the gene or the protein more important? Free Radic Biol Med. 2004;37:1317–1323.
- Jarvik GP, Rozek LS, Brophy VH, Furlong CE Paraoxonase (PON1) phenotype is a better predictor of vascular disease than is PON1(192) or PON1(55) genotype. Arterioscler Thromb Vasc Biol. 2000;20:2441–2447.
- Mackness B, Durrington P, Mc Elduff P, Low paraoxonase activity predicts coronary events in the Caerphilly Prospective Study. Circulation. 2003;107:2775–2779.
- Sarandol A, Sarandol E, Eker SS, Oxidation of apolipoprotein B-containing lipoproteins and serum paraoxonase/arylesterase activities in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1103–1108.
- Gomez-Farices MA, McClellan W, Soucie JM, Mitch WE. A prospective comparison of methods for determining if cardiovascular disease is a predictor of mortality in dialysis patients. Am J Kidney Dis. 1994;23:382–388.
- Owen WF, Madore F, Brenner BM. An observational study of cardiovascular characteristics of long-term end-stage renal disease survivors. Am J Kidney Dis. 1996;28:931–936.
- Girotti AW. Lipid hydroperoxide generation, turnover, and effector action in biological systems. J Lipid Res. 1998;39:1529–1542.
- Isik A, Koca SS, Ustundag B, Celik H, Yildirim A. Paraoxonase and arylesterase levels in rheumatoid arthritis. Clin Rheumatol. 2007;26:342–348.
- Gorog P, Kotak DC, Kovacs IB. Simple and specific test for measuring lipid peroxides in plasma. J Clin Pathol. 1991;44:765–767.
- National Kidney Foundation. Clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:1–266.
- Oberg BP, McMenamin E, Lucas FL, Increased prevalence of oxidant stress and inflammation in patients with moderate to severe chronic kidney disease. Kidney Int. 2004;65:1009–1016.
- Vanholder R, Argıles A, Baurmeıster U, Uremic toxicity: Present state of the art. Int J Artif Organs. 2001;24:695–725.
- Dubourg L, Mıchoudet C, Cochat P, Baverel G. Human kidney tubules detoxify chloroacetaldehyde, a presumed nephrotoxic metabolite of ifosfamide. J Am Soc Nephrol. 2001;12:1615–1623.
- Mıhoudet C, Baverel G. Metabolism of acetaldehyde in human and baboon renal cortex. FEBS Lett. 1987;216:113–117.
- Stuveling EM, Hillege HL, Bakker SJ, C-reactive protein is associated with renal function abnormalities in a non-diabetic population. Kidney Int. 2003;63:654–661.
- Pecoits-Filho R, Heimburger O, Barany P, Associations between circulating inflammatory markers and residual renal function in CRF patients. Am J Kidney Dis. 2003;41:1212–1218.
- Panichi V, Migliori M, De Pietro S, C-reactive protein and interleukin-6 levels are related to renal function in predialytic chronic renal failure. Nephron. 2002;91:594–600.
- Descamps-Latscha B, Herbelın A, Nguyen AT, Balance between IL-1 beta, TNF-alpha, and their specific inhibitors in chronic renal failure and maintenance dialysis: Relationships with activation markers of T cells, B cells, and monocytes. J Immunol. 1995;154:882–892.
- Dantoine TF, Debord J, Charmes JP, Decrease of serum paraoxonase activity in chronic renal failure. J Am Soc Nephrol. 1998;9:2082–2088.
- Schiavon R, De Fanti E, Giavarina D, Biasioli S, Cavalcanti G, Guidi G. Serum paraoxonase is decreased in uremic patients. Clin Chim Acta. 1996;247:71–80.
- Bolton CH, Downs LG, Victory JGG, Endothelial dysfunction in chronic renal failure: Roles of lipoprotein oxidation and pro-inflammatory cytokines. Nephrol Dial Transplant. 2001;16:1189–1197.
- Mezzano D, Pais EO, Aranda E, Inflammation, not hyperhomocysteinemia, is related to oxidative stress and hemostatic and endothelial dysfunction in uremia. Kidney Int. 2001;60:1844–1850.
- Witko-Sarsat V, Friedlander M, Nguyen-Khoa T, Advanced oxidation protein products as novel mediators of inflammation and monocyte activation in chronic renal failure. J Immunol. 1998;161:2524–2532.