Abstract
In this prospective randomized trial, we compared the effects of cyclosporine- and cyclophosphamide-based treatment regimens in patients with idiopathic membranous nephropathy. Twenty-eight patients were randomized to receive treatment with one of the three therapeutic regimens: cyclosporine with methylprednisolone, cyclophosphamide with methylprednisolone or lisinopril (control). Renal function and nephrotic syndrome parameters were determined at baseline and during a 9-month treatment period. At the end of the study period, renal function improved significantly in the cyclophosphamide and deteriorated significantly in the cyclosporine group. Serum albumin levels increased significantly in the cyclosporine and cyclophosphamide group. Total cholesterol levels and proteinuria were significantly reduced in all groups. In the comparison between the groups, serum albumin levels were significantly lower in the control group and there were no differences in the rest of the studied parameters at the end of the study. Six patients from the cyclosporine group (1/10 complete and 5/10 partial), all cyclophosphamide-treated (4/8 complete and 4/8 partial) and all 10 lisinopril-treated patients (10/10 partial) were on remission at the end of the study. In conclusion, cyclosporine-based regimens are not inferior to cyclophosphamide-based regimens. Cyclophosphamide is associated with more complete remissions after 9 months of treatment. Lisinopril is associated with a significant proteinuria reduction and without inducing any complete remissions.
INTRODUCTION
Idiopathic membranous nephropathy (IMN) is the most frequent cause of nephrotic syndrome (NS) in adults.Citation1 It is not considered a benign disease. In the Caucasian population, end-stage renal disease develops in 20–40% of patients at 10–15 years.Citation2 NS may spontaneously remit in about 20% of IMN cases, usually during the first 6–12 months after the initiation of the symptoms.Citation3
Even though significant breakthroughs have been achieved in the study of the etiopathogenic factors during the last years, the treatment of IMN remains a controversial issue. It is clear that the induction of remission is associated with the long-term renal prognosis.Citation4 Various therapeutic regimens have been introduced and the treatment of IMN mainly consists of the nonspecific “symptomatic” therapy – usually renin–angiotensin axis inhibitors and cholesterol-lowering agents – and the specific “immunosuppressive” treatment options.Citation5
The aim of this study was to compare the effects of a cyclosporine- and cyclophosphamide-based treatment regimen in patients with IMN and NS.
PATIENTS AND METHODS
Treatment protocol
In this prospective randomized trial, 30 treatment-naïve patients were initially recruited and randomized for the study and 28 of them (17 males, mean age ± SEM 52.4 ± 2.8 years) started treatment with three therapeutic regimens. The remaining two were excluded from the study after the recruitment, consent, and randomization but before the treatment initiation due to loss of follow-up for one patient and severe/fast deterioration of the renal function in another patient.
The first group (CYAMP) (10 patients, 8 males, mean age ± SEM 50.5 ± 4.9 years) was treated with 3–3.5 mg/kg/day oral cyclosporine and 12.5 mg/day oral methylprednisolone; the second group (CPSMP) (8 patients, 4 males, mean age ± SEM 55.4 ± 2.8 years) was treated with 2 mg/kg/24 hour oral cyclophosphamide and 1.5 mg/kg/48 hour oral methylprednisolone; and the control group (LIS) (10 patients, 5 males, mean age ± SEM, 51.8 ± 5.4 years) was treated with the angiotensin-converting enzyme inhibitor (ACEI) lisinopril.
The aim of this prospective randomized trial was to exclude the superiority of one regimen over the others (non-inferiority study). All groups received treatment with the studied regimens for a period of 9 months. Every new patient who was satisfying the inclusion criteria (biopsy-proven IMN with NS for a period over 6 months and no apparent secondary cause of membranous nephropathy, excluded after a thorough clinical and paraclinical screening) was randomized to receive one of the three test schemes. The person doing the randomization was blinded, that is, did not have the right to recruit and did not have direct contact with any of the patients. The recruiting and treating doctors as well as the patients were not blinded on the type of treatment throughout the medication period. At the beginning, after the first, third, sixth month and at the end of the study period (9 months), the glomerular filtration rate (GFR) using the four-component MDRD formula, GFR (mL/min/1.73 m2) = 175 × (Scr)exp −1.154 × (Age)exp −0.203 × (0.742 if female) × (1.212 if African American), serum albumin, total serum cholesterol as well as 24-hour proteinuria were determined. We tried to minimize the possibility of a spontaneous remission during the study period by recruiting and randomizing the patients 6 months after the histological confirmation of IMN as it was assumed that possible spontaneous remission could take place during this period of time. The study was approved by the ethics committee of the hospital and an informed consent form was explained, read, and signed by every participating patient after a comprehensive period of time for consideration.
General management
Patients with prior history of essential hypertension were excluded from the study.
In addition to the test drugs, patients were placed on a low-sodium diet (5 g/day) and given moderate doses of loop diuretics if indicated, as well as anti-hypertensive agents (beta-blockers and/or dihydropyridine calcium-blockers) if blood pressure readings were above 140/90 mmHg. ACEIs and angiotensin receptor blockers were not prescribed to the CYAMP and the CPSMP patients. Cyclophosphamide dose was adjusted according to the leukocyte count. The daily dose of cyclosporine was adjusted to trough levels ranging from 100 to 120 μg/L. Concerning the prevention of steroid toxicity, all patients underwent initial and follow-up orthopedic and eye examinations. In lisinopril-treated patients, renal function and potassium levels were followed closely and low potassium diet was prescribed if the serum potassium levels were abnormally high.
Definitions
Nephrotic patients were identified by a proteinuria value ≥3.5 g/day associated with edema, hypoalbuminemia, and hypercholesterolemia. A complete remission was defined by a proteinuria value ≤0.3 g/day. A partial remission was defined by a proteinuria value <3.5 g/day plus a 50% reduction from its peak value.
Data and study protocols – statistical analysis
Data were obtained at the start and at the end of the first, third, sixth, and ninth months of the treatment period. This is a non-superiority study comparing the two most common immunosuppressive regimens in patients with IMN and NS. The null hypothesis is that these two regimens do not differ significantly in proteinuria reduction after 12 months of treatment. The primary end-point is the proteinuria reduction at the end of the study and the secondary end-points are the rate of change of the renal function (estimated GFR), as well as the change of other parameters of the NS (serum albumin, total cholesterol, and 24-hour proteinuria). We compared the levels of the studied parameters in the three groups and between the three groups throughout the medication period.
Values are given as mean ±SEM. Statistical analysis was performed using the SPSS® Version 17.0 (SPSS Inc., Chicago, IL, USA). ANOVA was performed to evaluate the overall significance in the rate of change for the studied parameters in the course of the values during the treatment period. A paired sample t-test was performed to compare the timed values of every studied parameter with the baseline ones. ANOVA was also performed to test the timing effect of the studied parameters between the three groups during the study. A post hoc analysis (Bonferroni analysis) was performed to compare the differences between the three groups in the studied parameters at every specific time-point throughout the study. p-Values less than 0.05 were considered to be significant.
RESULTS
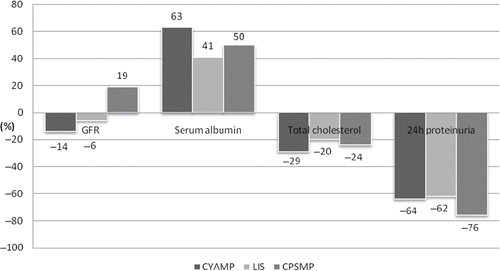
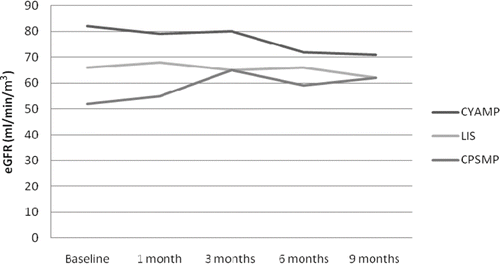
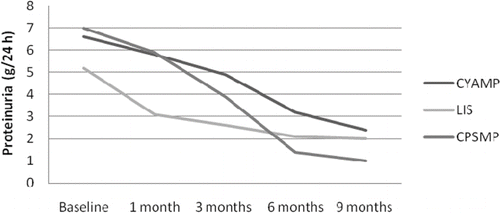
Concerning the baseline values () there was a significant difference in the estimated GFR (eGFR) values between the three groups. Baseline proteinuria, total cholesterol, and serum albumin were not different between the study groups (). Concerning the CYAMP group, eGFR gradually deteriorated in time and became significant at the end of the 9-month therapy period [mean levels ± SEM (mL/min/m3) – baseline (82 ± 8) vs. end of study (71 ± 8), p =0.034] (, ). Serum albumin in the CYAMP group was increased significantly from the first month and remained so until the end of the treatment period [mean levels ± SEM (g/L) – baseline (2.7 ± 0.2) vs. end of study (4.2 ± 0.1), p <0.0001] and total cholesterol was significantly reduced from the sixth month until the end of the study period [mean levels ± SEM (mg/dL) – baseline (403 ± 41) vs. end of study (287 ± 19), p =0.005] (, ). Twenty-four-hour proteinuria was significantly reduced in this group from the first month and until the end of the treatment period [mean levels ± SEM (g/24 h) – baseline (6.6 ± 1.0) vs. end of study (2.4 ± 0.5), p =0.003] (, ). In the CPSMP group, eGFR was significantly increased by the end of the study [mean levels ± SEM (mL/min/m3) – baseline (52 ± 7) vs. end of study (62 ± 6), p =0.033] (, ). Serum albumin was increased significantly from the third month [mean levels ± SEM (g/L) – baseline (2.8 ± 0.2) vs. end of study (4.2 ± 0.2), p =0.001]; total cholesterol was significantly reduced from the sixth month of the study [mean levels ± SEM (mg/dL) – baseline (377 ± 21) vs. end of study (285 ± 26), p =0.017]; and 24-hour proteinuria reduction became significant from the first month [mean levels ± SEM (g/24 h) – baseline (7 ± 0.7) vs. end of study (1.0 ± 0.4), p <0.0001] (, ). Concerning the control group there were no changes in eGFR throughout the study [mean levels ± SEM (mL/min/m3) – baseline (66 ± 6) vs. end of study (62 ± 6), p =0.556] (, ); albumin and 24-hour proteinuria significantly improved from the third month [albumin mean levels ± SEM (g/L) – baseline (2.2 ± 0.1) vs. end of study (3.1 ± 0.2), p <0.0001] and 24-hour proteinuria [mean levels ± SEM (g/24 h) – baseline (5.2 ± 0.8) vs. end of study (2.0 ± 0.2), p =0.002] (, ); and total cholesterol was significantly reduced from the sixth month of the study period [mean levels ± SEM (mg/dL) – baseline (355 ± 28) vs. end of study (285 ± 23), p =0.002] (, ). In the comparisons between groups, at the end of the study serum albumin levels were significantly lower in the lisinopril-control group compared to the other study groups (). There were no significant differences at the end of the study between the three groups concerning proteinuria, serum albumin, and total cholesterol ().
TABLE 1. Comparison of the baseline characteristics between the three study groups
TABLE 2. Comparison of the end of study characteristics between the three study groups
TABLE 3. Comparison of the baseline and the end of study characteristics in the three study groups
At the end of the treatment period, 60% (six patients) from the cyclosporine-treated group were on remission (1/10 complete and 5/10 partial). At the end of the medication period, all cyclophosphamide-treated patients were on remission (4/8 complete and 4/8 partial) and all 10 lisinopril-treated patients were on partial remission.
The complications were not frequent and did not affect seriously the course of the study. In two of the cyclophosphamide-treated patients a transient leucopenia was observed with a total white blood count just under 3000/mm3and a Herpes Zoster infection successfully treated with antivirals. From the lisinopril-treated group, two patients presented with hyperkaliemia (serum potassium levels over 6 meq/L) and two patients with symptomatic hypotension.
DISCUSSION
This study investigated the comparative effects of two common immunosuppressive regimens in the treatment of IMN. The control group was treated “symptomatically” with an ACEI. It was considered unethical by the investigators to treat the control group with placebo or no therapeutic agent at all. Historically, the first attempts for the specific treatment of IMN were done with the use of corticosteroids. Later on, cytotoxic agents were proved more efficient. A meta-analysis of studies on the treatment for IMN showed that cytotoxic agents with or without corticosteroids increased fourfold the probability of a remission compared to corticosteroids given alone or symptomatic therapy only.Citation6,Citation7
Therapeutic regimens with chlorambucil and corticosteroids given in alternate months (the Ponticelli scheme) proved the beneficial effects of cytotoxic agents in IMN. Chlorambucil was later replaced by cyclophosphamide given intravenously every month or per os in longer and shorter timed courses.Citation8
Later on, calcineurin inhibitor cyclosporine was introduced in the treatment of IMN with or without corticosteroids and it was found to be effective but with an increased rate of relapses as well as nephrotoxicity after the completion of therapy. Intravenous immunoglobulin, azathioprine, mycophenolate mofetil, tacrolimus, and rituximab have also been used successfully in the treatment of IMN.Citation5 During the last two decades, the majority of patients have been treated with cyclosporine-based or cyclophosphamide-based treatment regimens. It is known that up to 40% of untreated patients with IMN eventually develop end-stage renal diseaseCitation5 and patients on complete remission have a better long-term renal prognosis.Citation4 In view of these facts, it is important to try inducing and maintaining remission in patients with IMN and NS, especially the ones with relatively bad prognostic factors from a clinical (diuretic-resistant generalized edema), biochemical (deterioration of the renal function, very severe proteinuria, associated with severe hypolipidemia and hypoalbuminemia), and histological (signs of tubulointerstitial fibrosis) point of view.
In this study, the renal function of the cyclosporine group deteriorated significantly during the study period, possibly because of intrarenal vasoconstriction and calcineurin inhibitor nephrotoxicity.Citation9 On the other hand, renal function was significantly improved in the cyclophosphamide-treated patients, an important finding that has been observed in earlier studies ().Citation10
The results of this study bring into dispute the influence of immunosuppression in patients with IMN and NS and the debate on the use of cyclophosphamide or cyclosporine. The present results are in agreement with those of a big meta-analysis by Remuzzi et al. in which there were no differences when data from all treatment categories were combined as a group and compared with placebo or no treatment and there was no evidence of clinically relevant differences in favor of cyclosporine.Citation11
Remarkably, treatment with ACEI was associated with significant improvement in the parameters of NS without affecting significantly the renal function.Citation12 Renin–angiotensin axis inhibition is attracting the interest of the treating doctors. It is believed that combination therapy with two or three agents aimed to inactivate the renin–angiotensin axis may have an additive effect on NS parameters of nondiabetic renal patients.Citation13
Treatment with lisinopril led to a reduction of the proteinuria levels by 62%. This is a useful finding that coincides with the GISEN group findings (ACEI ramipril significantly reduced proteinuria levels in nondiabetic nephrotic patients).Citation14 Other studies have indicated modest antiproteinuric effects (up to 30%) in patients with IMN and NS treated with ACEI.Citation15 The patient population was not different in some important way from that in the earlier studies.
In this study, all treatment options were associated with a remarkable improvement in the parameters of the NS and the response was significant from the earlier stages of the treatment period, usually from the end of the first or the third month of treatment (). We believe that this is a useful finding because it can be used as a tool for the assessment of response to therapy. As indicated in previous studies, a possible lack of significant reductions in NS parameters 3 months after the initiation of the treatment may, with a fair level of certainty, lead to a rescheduling of the therapeutic strategy.Citation16 A weak point of this study is that it is prospective randomized and not double-blind randomized. Nevertheless, it should be noted that every new patient who was fulfilling the inclusion criteria was randomized by a “blinded” person who was not involved in the recruitment or the treatment of the patients and had no contact with them. Additionally, the investigators had no external funding for this study and no reason to be biased for or against a specific substance.
FIGURE 3. Mean 24-hour proteinuria levels of the three patient groups in different time-points of the study.
In conclusion, CYAMP, MP, and CPSMP therapy in patients with NS due to IMN improves similarly hypoalbuminemia, hypercholesterolemia, and proteinuria without changing renal function.
Declaration of Interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Mason PD, Pusey CD. Glomerulonephritis: Diagnosis and treatment. BMJ. 1994;309(6968):1557–1563.
- Honkanen E, Törnroth T, Grönhagen-Riska C. Natural history, clinical course and morphological evolution of membranous nephropathy. Nephrol Dial Transplant. 1992;(Suppl. 1):35–41.
- Ponticelli C, Passerini P. Membranous nephropathy. In: Ponticelli C, Glassock RJ, eds. Treatment of Primary Glomerulonephritis. Oxford: Oxford University Press; 1997:146–185.
- Passerini P, Pasquali S, Cesana B, Zucchelli P, Ponticelli C. Long-term outcome of patients with membranous nephropathy after complete remission of proteinuria. Nephrol Dial Transplant. 1989;4:525–529.
- Quaglia M, Stratta P. Idiopathic membranous nephropathy: Management strategies. Drugs. 2009;69(10):1303–1317.
- Hogan SL, Muller KE, Jennette JC, Falk RJ. A review of therapeutic studies of idiopathic membranous glomerulopathy. Am J Kidney Dis. 1995;25:862–875.
- Imperiale TF, Goldfarb S, Berns JS. Are cytotoxic agents beneficial in idiopathic membranous nephropathy? A meta-analysis of the controlled trials. J Am Soc Nephrol. 1995;5:1553–1558.
- Fujimoto S, Hara S, Sato Y, Yamada K, Hisanaga S, Eto T. Nephrotic syndrome caused by membranous nephropathy: Response to a short course of cyclophosphamide alternating with prednisolone. Intern Med. 2004;43(1):30–34.
- Goumenos DS, Kalliakmani P, Tsakas S, Sotsiou F, Vlachojannis JG. The remission of nephrotic syndrome with cyclosporin treatment does not attenuate the progression of idiopathic membranous nephropathy. Clin Nephrol. 2004;61(1):17–24.
- Branten AJ, Reichert LJ, Koene RA, Wetzels JF. Oral cyclophosphamide versus chlorambucil in the treatment of patients with membranous nephropathy and renal insufficiency. QJM. 1998;91(5):359–366.
- Schieppati A, Perna A, Zamora J, Giuliano GA, Braun N, Remuzzi G. Immunosuppressive treatment for idiopathic membranous nephropathy in adults with nephrotic syndrome. Cochrane Database Syst Rev. 2004;18(4):CD004293.
- Praga M, Hernández E, Montoyo C, Andrés A, Ruilope LM, Rodicio JL. Long-term beneficial effects of angiotensin-converting enzyme inhibition in patients with nephrotic proteinuria. Am J Kidney Dis. 1992;20(3):240–248.
- Tylicki L, Rutkowski P, Renke M, Triple pharmacological blockade of the renin-angiotensin-aldosterone system in nondiabetic CKD: An open-label crossover randomized controlled trial. Am J Kidney Dis. 2008;52(3):486–493.
- The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia). Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. Lancet. 1997;349(9069):1857–1863.
- Ruggenenti P, Mosconi L, Vendramin G, ACE inhibition improves glomerular size selectivity in patients with idiopathic membranous nephropathy and persistent nephrotic syndrome. Am J Kidney Dis. 2000;35:381–391.
- Gansevoort RT, Heeg JE, Vriesendorp R, de Zeeuw D, de Jong PE. Antiproteinuric drugs in patients with idiopathic membranous glomerulopathy. Nephrol Dial Transplant. 1992;7(Suppl. 1):91–96.