Abstract
Background: A unique form of postinfectious glomerulonephritis (PIGN) with IgA-dominant deposition mimicking IgA nephropathy has been increasingly reported. Methods: We compared the clinical and histological features of 12 patients with postinfectious IgA-dominant glomerulonephritis to 134 patients with idiopathic IgA nephropathy. Results: In addition to hypocomplementemia and subepithelial hump-shaped deposits characteristic of PIGN, patients with postinfectious IgA-dominant glomerulonephritis had older age (62.3 ± 16.9 vs. 37.9 ± 16.3 years; p < 0.001) and more frequently presented with acute renal failure (83.3% vs. 10.4%; p < 0.001) than patients with idiopathic IgA nephropathy. Moreover, glomerular changes including endocapillary proliferation, neutrophil infiltration, and capillary loops deposits by immunofluorescence were more commonly present in postinfectious IgA-dominant glomerulonephritis group (p < 0.001). Conclusions: PIGN could be characterized by glomerular IgA-dominant deposition resembling idiopathic IgA nephropathy. It is essential to differentiate postinfectious IgA-dominant glomerulonephritis from idiopathic IgA nephropathy because of the different treatments and prognosis of the two diseases.
INTRODUCTION
Glomerular IgA-dominant deposition in the setting of proliferative glomerulonephritis (GN) usually indicates IgA nephropathy, Henoch–Schönlein nephritis, or, less often, lupus nephritis; and it is rare and not predominant in postinfectious glomerulonephritis (PIGN). Recently, however, there have been several reports of PIGN with IgA-dominant immune complex deposition, mostly related to staphylococcal infection.Citation1–5 At our institution, we also have encountered several cases of PIGN in which renal biopsies showed IgA-dominant deposition strongly resembling idiopathic IgA nephropathy. It is important to differentiate postinfectious IgA-dominant GN from idiopathic IgA nephropathy to avoid incorrect treatment with immunosuppressive medications. Therefore, we conducted this study to elucidate the clinicopathological characteristics of postinfectious IgA-dominant GN and its discrimination from idiopathic IgA nephropathy.
METHODS
Between January 2000 and June 2009, we identified 12 patients with PIGN whose renal biopsies showed glomerular predominant or codominant IgA deposits. All patients had no history of renal disease previously and had culture-documented bacterial infection before the occurrence of renal manifestations. As a comparison group, we included 134 cases of idiopathic IgA nephropathy that were identified during the same period. We excluded patients with clinical or serological evidence for systemic lupus erythematosus, Henoch–Schönlein purpura, or chronic liver disease. Patients' medical records were reviewed for age, gender, type, and source of infection, degree of hematuria, serum creatinine at the time of biopsy, urinary protein excretion (g/24 h or protein/creatinine ratio), and outcome. We defined acute renal failure as an increase of serum creatinine of at least 0.5 mg/dL above baseline values. Scoring of hematuria was based on the number of red blood cells per high power field in sediment and was graded as 0 if 0–5, 1 if 6–20, 2 if 21–50, 3 if 51–100, and 4 if >100. The extent of mesangial hypercellularity was scored as 1+ if involving <25% of glomeruli, 2+ if involving 25–50%, and 3+ if involving ≥50%. The chronic tubulointerstitial injury presented as tubular atrophy and interstitial fibrosis was graded as mild if involving <25%, moderate if involving 25–50%, and severe if involving ≥50%. The intensity of immunofluorescence staining was also graded on a scale of 0 to 3+. For outcome analysis, remission of acute renal failure was defined as the normalization of serum creatinine to baseline levels or to ≤1.2 mg/dL for those patients in whom baseline levels of creatinine were unavailable; and renal insufficiency was defined by elevation of serum creatinine above baseline levels or follow-up creatinine >1.2 mg/dL for those patients in whom baseline levels of creatinine were unavailable. End-stage renal disease (ESRD) was defined as requiring maintenance dialysis therapy.
Statistical analyses were performed using SPSS version 12 (SPSS Inc., Chicago, Illinois, USA) for Windows and produced by simple nonparametric test (Mann–Whitney U-test and Fisher's exact test). A p-value <0.05 was considered significant.
RESULTS
The demographic and clinical features of the 12 patients with postinfectious IgA-dominant GN are summarized in . There were 9 males and 3 females. The mean age was 62 ± 17 years (range 23–80 years). All patients had hematuria (score 4) and macroscopic hematuria was present in 4 patients. The mean proteinuria was 3.9 ± 3.2g/24 h (range 0.4–9.8 g/24h). The mean serum creatinine at the time of biopsy was 5.0 ± 3.5 mg/dL (range 0.6–11.8 mg/dL). The great majority of patients (10 out of 12) had acute renal failure at presentation, and half of them required dialysis support. Hypocomplementemia was present in 7 out of 12 patients (58%). Increased serum IgA levels were noted in 6 out of 9 patients (67%). The two most frequently identified infectious pathogens were Staphylococcus (7 patients) and Streptococcus (2 patients). The remaining 3 were inflicted with Gram-negative bacteria including 1 Klebsiella pneumoniae, 1 Escherichia coli, and 1 Acinetobacter baumannii. In the 7 patients with staphylococcal infection, 3 had methicillin-resistant Staphylococcus aureus (MRSA), 3 had methicillin-sensitive Staphylococcus aureus (MSSA), and 1 had methicillin-sensitive Staphylococcus epidermidis (MSSE). Except three patients who died within 3 months after diagnosis (two died of pneumonia and one died of central line catheter-related infection), the remaining nine patients were followed for a mean of 27 ± 23 months. Seven patients entered remission, one had renal insufficiency, and one with ESRD had to be submitted to chronic dialysis.
TABLE 1. Demographic and clinical features of patients with postinfectious IgA-dominant glomerulonephritis
lists the summary of the 12 patients with postinfectious IgA-dominant GN. Four biopsies showed diffuse endocapillary proliferative GN with prominent neutrophil infiltration. The remaining eight biopsies showed predominantly mesangial hypercellularity with sparse glomerular neutrophils. Glomerular crescents were present in four patients (range 11–71%, affected ≥50% of glomeruli in one patient). Two biopsies showed focal glomerular tuft necrosis. In our series, one patient (case 8) had diabetes and whose renal biopsy showed underlying diabetic glomerulosclerosis. The mean percentage of globally sclerotic glomeruli was 16 ± 11% (range 0–36%). Acute tubular injury with luminal ectasia, epithelial simplification, loss of brush border, and nuclear enlargement was identified in seven patients. All biopsies showed mild chronic interstitial injury. All but one biopsy showed interstitial inflammation composed mainly of mononuclear leukocytes. The majority was focal and only one (case 4) was diffuse. In addition to IgA, all biopsies showed glomerular granular staining for C3 by immunofluorescence. Among them, two were in both mesangial areas and glomerular capillary loops, six were limited to mesangial areas, and four were limited to glomerular capillary loops (). On electron microscopy, subepithelial electron-dense deposits were present in five patients, and characteristic large hump-shaped deposits were seen in four patients ().
TABLE 2. Histological features of patients with postinfectious IgA-dominant glomerulonephritis
FIGURE 1. Immunofluorescence staining for IgA in a patient with diffuse endocapillary proliferative glomerulonephritis shows 2+ granular deposition in predominantly glomerular capillary loops (original magnification ×400); there is also 2+ staining for C3 in a similar pattern (not shown).
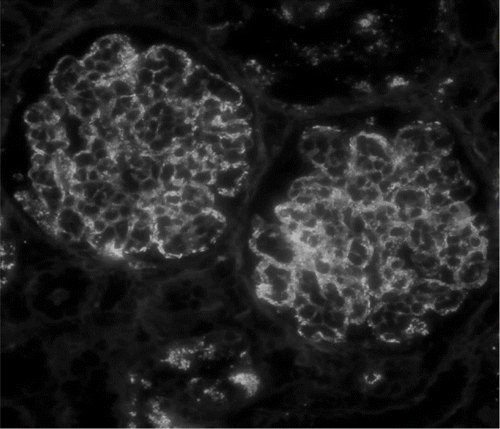
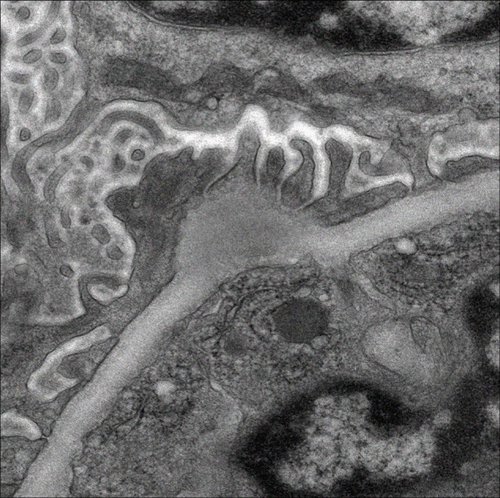
FIGURE 2. Electron microscopy shows large subepithelial hump-shaped electron dense deposition (original magnification × 8000).
lists the comparison of clinicopathological parameters between postinfectious IgA-dominant GN and idiopathic IgA nephropathy. In addition to hypocomplementemia and subepithelial hump-shaped deposits characteristic of PIGN, patients with postinfectious IgA-dominant GN had older age (62.3 ± 16.9 vs. 37.9 ± 16.3 years, p < 0.001), a higher degree of hematuria (score 4 ± 0 vs. 2.0 ± 1.5, p < 0.001), a higher incidence of increased serum IgA levels (66.7% vs. 33.3%, p = 0.068), more severe renal failure at the time of biopsy (serum creatinine 5.0 ± 3.5 vs. 2.2 ± 2.6 mg/dL, p < 0.001), and more frequently presented with acute renal failure (83.3% vs. 10.4%, p < 0.001) than the patients with idiopathic IgA nephropathy. Moreover, glomerular changes including endocapillary proliferation, neutrophil infiltration, and capillary loops deposits by immunofluorescence were more commonly present in postinfectious IgA-dominant GN group (p < 0.001).
TABLE 3. Comparison of clinicopathological parameters between postinfectious IgA-dominant glomerulonephritis and idiopathic IgA nephropathy
DISCUSSION
There is a broad spectrum of glomerular histological findings in PIGN.Citation6,Citation7 The typical histological pattern of PIGN includes diffuse endocapillary proliferative GN with infiltrating neutrophils within the capillary lumens (exudative features) on light microscopy, C3 granular deposition (often with IgG and occasionally with IgM) in a predominantly capillary wall distribution on immunofluorescence, and characteristic subepithelial hump-shaped deposits on electron microscopy. IgA deposition, if ever present, is usually not predominant.Citation8 The more extensive use of renal biopsy has demonstrated the presence of atypical histological features of PIGN. Recently, a unique form of PIGN characterized by IgA-dominant deposits was recently described by Nasr et al.Citation1 They reported five cases of IgA-dominant PIGN with typical diffuse endocapillary proliferation with exudative features; and subepithelial hump-shaped deposits could be identified in all patients. Each occurred after staphylococcal infection (3 MSSA, 2 S. epidermidis) in patients with underlying diabetic nephropathy. There have been other small series studies of PIGN with IgA-containing immune complex deposits in which most cases were associated with staphylococcal infection, although the histological features of these cases were quite variable. Koyama et al. reported 10 patients with PIGN occurring after MRSA infection whose renal biopsies showed various types of IgA-codominant GN including segmental necrotizing GN, focal-to-diffuse mesangial proliferative GN, and diffuse endocapillary proliferative GN.Citation2 Nagaba et al. reported eight cases of IgA-dominant PIGN occurring after MRSA infection in which two distinct patterns of morphology were described: four necrotizing crescentic GN and four mesangial proliferative GN.Citation3 Satoskar et al. reported eight patients with IgA-dominant PIGN and underlying staphylococcal infection (5 MRSA, 2 MSSA, 1 methicillin-resistant S. epidermidis) whose renal biopsies showed mild-to-moderate mesangial proliferative GN in six patients and prominent endocapillary proliferative GN in two patients.Citation4 Of these studies, however, electron microscopy often was not done or did not to show subepithelial hump-shaped deposits typical of PIGN. More recently, Haas et al. reported 13 cases of PIGN with ultrastructurally characteristic subepithelial hump-shaped deposits in which six were inflicted with staphylococcal infection (3 MRSA, 3 MSSA), two was HIV-positive, one was hepatitis C-positive, and four had no documented infection history.Citation5 Among them, five had diffuse endocapillary proliferative GN and eight had focal-to-diffuse mesangial proliferative GN. In our series, seven patients were inflicted with staphylococcal infection (3 MRSA, 3 MSSA, 1 MSSE) and five patients were infected with nonstaphylococcal infection (2 streptococci, 3 Gram-negative bacteria). On histology, four patients had diffuse endocapillary proliferative GN and eight patients had mild mesangial proliferative GN. Ultrastructural finding of subepithelial hump-shaped deposits was present in four patients and was exclusively found in patients with diffuse endocapillary proliferative GN.
The different histological features may represent different stages of PIGN: diffuse endocapillary and, to a lesser degree, mesangial proliferation with prominent neutrophil infiltration and subepithelial humps in the acute phase, less prominent endocapillary proliferation with loss of neutrophils and early resorption of deposits in the subacute phase, and mainly mesangial proliferation with extensive resorption of subepithelial humps in the resolving phase.Citation5,Citation8,Citation9 The histological findings largely depend on the timing of renal biopsy. In general practice, renal biopsy is not always mandatory during acute phase of infectious diseases complicated by renal dysfunction since treatment of underlying infectious diseases is still the mainstay therapy even though PIGN is confirmed by renal biopsy. Furthermore, the acute phase of PIGN itself may be subclinical, detected only on a renal biopsy enthusiastically performed because of asymptomatic renal dysfunction or microscopic urinary abnormalities. Thus, these may account for a great number of PIGN patients in our and other series reports whose renal biopsy showed mesangial proliferation, rather than endocapillary proliferation, without subepithelial humps.
Another question remain to be answered is why these PIGN patients had prominent IgA deposition. It has been speculated that bacterial superantigen played an important role in the pathogenesis of this unique form of PIGN. Koyama et al. indicated that staphylococcal enterotoxins may behave as superantigens that can bind directly to major histocompatibility complex (MHC) class II molecules on antigen-presenting cells.Citation2 The enterotoxin/MHC class II complex then binds to the T cell receptor Vβ region without MHC restriction. They proposed that these processes results in massive T cell activation with subsequent cytokine burst. The cytokines activate B cells that will produce polyclonal IgA and IgG, which would eventually result in immune complex formation. Recently, Koyoma et al. proposed a particular S. aureus cell envelope antigen as the pathogenetic protein.Citation10 They were able to co-localize the antigen with the glomerular IgA deposits in the glomeruli of affected patients. Also, these workers recently developed an experimental model in Balb/c mice after immunization of the animals with this S. aureus wall antigen.Citation11,Citation12 The animals developed mesangial IgA deposits. Regarding nonstaphylococcal pathogens, Endo et al. demonstrated that various strains of gram-negative bacteria cell wall components, including Pseudomonas aeruginosa, E. coli, Hemophilus influenzae, and K. pneumoniae, can induce glomerular deposition of IgA and C3 in animal study.Citation13
On histology, IgA-dominant PIGN is difficult to differential from idiopathic IgA nephropathy, in particular with resolving lesions presenting with mesangial proliferation with mesangial pattern of immune deposits by immunofluorescence and loss of endocapillary proliferation, neutrophil infiltration and subepithelial humps. In our experience, clinical findings favor IgA-dominant PIGN over idiopathic IgA nephropathy include older age, decreased serum complement levels, intercurrent infection, and acute renal failure at presentation (). It is important to discriminate between the two conditions because of the different treatments and prognosis.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Nasr SH, Markowitz GS, Whelan JD, IgA-dominant acute post staphylococcal glomerulonephritis complicating diabetic nephropathy. Hum Pathol. 2003;34:1235–1241.
- Koyama A, Kobayashi M, Yamaguchi N, Glomerulonephritis associated with MRSA infection: A possible role of bacterial superantigen. Kidney Int. 1995;47:207–216.
- Nagaba Y, Hiki Y, Aoyama T, Effective antibiotic treatment of methicillin-resistant Staphylococcus aureus-associated glomerulonephritis. Nephron. 2002;92:297–303.
- Satoskar AA, Nadasdy G, Plaza JA, Staphylococcus infection-associated glomerulonephritis mimicking IgA nephropathy. Clin J Am Soc Nephrol. 2006;1:1179–1186.
- Haas M, Racusen LC, Bagnasco SM. IgA-dominant postinfectious glomerulonephritis: A report of 13 cases with common ultrastructural features. Hum Pathol. 2008;39:1309–1316.
- Edelstein CL, Bates WD. Subtypes of acute postinfectious glomerulonephritis: A clinico-pathological correlation. Clin Nephrol. 1992;38:311–317.
- Sotsiou F, Dimitriadis G, Liapis H. Diagnostic dilemmas in atypical postinfectious glomerulonephritis. Semin Diagn Pathol. 2002;19:146–159.
- Nadasdy T, Silva FG. Acute postinfectious glomerulonephritis and glomerulonephritis caused by persistent bacterial infection. In: Jennette JC, Olson JL, Schwartz MM, Silva FG, eds. Heptinstall's Pathology of the Kidney. 6th ed. Philadelphia: Lippincott Williams & Wilkins; 2007:321–396.
- Kanjanabuch T, Kittikowit W, Eiam-Ong S. An update on acute postinfectious glomerulonephritis worldwide. Nat Rev Nephrol. 2009;5:259–269.
- Koyama A, Sharmin S, Sakurai H, Staphylococcus aureus cell envelope antigen is a new candidate for the induction of IgA nephropathy. Kidney Int. 2004;66:121–132.
- Sharmin S, Shimizu Y, Hagiwara M, Hirayama K, Koyama A. Staphylococcus aureus antigens induce IgA-type glomerulonephritis in Balb/c mice. J Nephrol. 2004;17:504–511.
- Shimizu Y, Sakurai H, Hirayama K, Staphylococcal cell membrane antigen, a possible antigen in post-methicillin resistant Staphylococcus aureus (MRSA) infection nephritis and IgA nephropathy, exhibits high immunogenic activity that is enhanced by superantigen. J. Nephrol. 2005;18:249–256.
- Endo Y, Kanbayashi H, Hara M. Experimental immunoglobulin A nephropathy induced by gram-negative bacteria. Nephron. 1993;65:196–205.
