Abstract
Objectives: Sepsis model was used to understand the role of sustained hyperglycemia and ovariectomy, either separately or concomitantly, on the response of the activity of the nuclear factor kappa B (NF-κB) and the oxidative response in kidney. Subjects: Polymicrobial sepsis was induced by cecal ligation and puncture (CLP). Diabetes was induced in female rats using administration of alloxan. The rats were divided into five groups: sham control (group 1), ovariectomy (group 2), ovariectomy + sepsis (group 3), ovariectomy + diabetes (group 4), and ovariectomy + diabetic + sepsis (group 5). Results: In kidney tissues, the levels of lipid peroxidation (LPO) and glutathione (GSH) and the activity of catalase (CAT) were higher for groups 3, 4, 5 than the control groups. Superoxide dismutase (SOD) activity was lower for groups 3, 4, 5 than the control groups. We determined that CLP produced injury evident in the kidneys of rats when compared to the control group, whereas the severity of the injury was higher in the diabetes + ovariectomy + CLP group when compared to the CLP group. In immunohistochemical staining, we determined that CLP operation increased NF-κB activation. In the ovariectomized, septic, and diabetic group, NF-κB activation was significantly higher than other groups. Conclusions: Hyperglycemia and ovariectomy severely increased NF-κB activation and oxidant levels with the stages of our sepsis model. Ovariectomy resulted in general changes in metabolism, which are seen in the kidney with diabetes under sepsis conditions.
INTRODUCTION
Sepsis can lead to multiple organ failure and is characterized by decomposition of the inflammatory response. This continues to be the most common cause of mortality and morbidity in intensive care units.Citation1 Sepsis gives rise to acute kidney injury (AKI) and patients with both sepsis and AKI have an especially high mortality rate. Severe sepsis, including renal and hepatic failure, occurs in 750,000 patients per year in the United States.Citation2 Sepsis is a contributing factor in 48% of patients with AKI.Citation3,Citation4 Nuclear factor kappa B (NF-κB) is an inducible nuclear transcription factor that plays a central role in regulating the transcription of several genes, including those that encode the proinflammatory cytokines, such as tumor necrosis factor α (TNF-α), interleukin-6(IL-6), adhesion molecules, and additional proinflammatory mediators, involved in severe sepsis, septic shock, acute respiratory distress syndrome (ARDS), and acute lung injury (ALI).Citation5,Citation6
Estrogens exert profound effects on the growth, differentiation, and function of many reproductive tissues. They also affect other tissues, including bone, liver, cardiovascular system, and brain. Recent studies have determined the association between oxidative status and gonadal hormones.Citation7,Citation8 The antioxidant enzyme system seems to be affected in this phase due to estrogen deficiency. Estrogen protects women against various diseases such as osteoporosis, atherosclerosis, and neurodegenerative diseases through various mechanisms.Citation9 The relative estrogen deprivation in postmenopausal women is associated with physiological changes such as an increased level of reactive oxygen species (ROS) and increased risk of several diseases, including cardiovascular disease and osteoporosis.Citation10,Citation11 The beneficial effects of estrogen might be attributable to its antioxidant properties.Citation12 Estrogen deficiency in postmenopausal women leads to oxidative stress and becomes the cause of various pathologies because of the release of free radicals or ROSs including superoxide, hydroxyl radical, hydrogen peroxide, peroxynitrite, hypochlorous acid, and lipid radicals.Citation13 Furthermore, in animal models, the bilateral ovariectomy was shown to impair insulin sensitivity and glucose metabolism. This is a harmful metabolic effect that is reversed by the chronic administration of estrogens.Citation14,Citation15 According to this data, short-term administration of estrogen was found to improve proteinuria and creatinine clearance in diabetic and hypertensive postmenopausal women.Citation16 Another study supports the beneficial effect of estrogens on insulin action, which was demonstrated to be greater in premenopausal women.Citation17,Citation18 In this study, an animal model of ovariectomy with sepsis and diabetes was used to investigate the effects on immunologic response, mortality, and tissue oxidant status.
Alloxan-induced diabetic rats are frequently used to study insulin-dependent diabetes in animal studies.Citation19 Diabetes mellitus (DM) is a metabolic disorder characterized by hyperglycemia, which is caused by insulin deficiency.
In evaluating the overall related literature, it is clear that there are not enough studies focusing on the effects of ovariectomy on diabetes and sepsis in the kidney tissues of rats. The rat intra-abdominal sepsis model is considered to be a simple and enforceable model in rats. In this study, this model was used to understand the role of sustained hyperglycemia and ovariectomy, either separately or concomitantly, on the response of NF-κB activation and oxidative response. The stated hypothesis was that ovariectomy may induce variations in antioxidant/oxidant status and in NF-κB activation detected in rat kidney tissues in animal models of diabetes and/or sepsis.
MATERIALS AND METHODS
Animals
In this study, 75, twelve-week-old, female Wistar rats were used for all experiments, each weighing 220–240 g. They were obtained from the Experimental Animal Laboratory of Medicinal and Experimental Application and Research Center at Ataturk University (ATADEM). Animal experiments and procedures were performed in accordance with the national guidelines for the use and care of laboratory animals and were approved by the local animal care committee at Ataturk University.
Chemicals
All chemicals for laboratory experimentation were purchased from Sigma Chemical Co. (Steinheim, Germany).
Experimental design
The rats were divided into five groups, each containing 15 rats: sham operated control (group 1), ovariectomy (group 2), ovariectomy + diabetes (group 3), ovariectomy + cecal ligation and puncture (CLP)-induced sepsis (group 4), and ovariectomy + diabetes + CLP (group 5). The groups were maintained separately in different cages. After ovariectomy surgery (in groups 2, 3, 4, and 5), all groups of rats were allowed to recover for 3 months (groups 1, 2, 3, 4, and 5). Groups 3 and 5 were administered alloxan for the induction of diabetes after the recovery period. Finally, all groups of rats were allowed to recover for 1 month (groups 1, 2, 3, 4, and 5) ().
TABLE 1. Experimental protocol of rat groups
Alloxan-induced diabetes
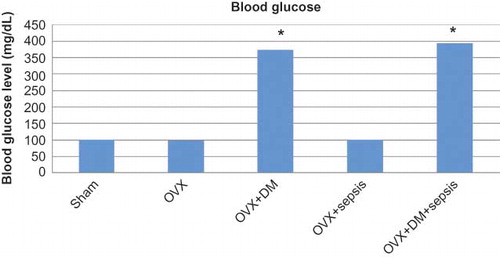
Diabetes was induced in the female Wistar albino rats by intraperitoneal administration of aqueous alloxan monohydrate (Sigma-Aldrich Co., Steinheim, Germany) at a single dose of 150 mg/kg body weight, as previously described.Citation20 Alloxan was dissolved in 0.9% NaCl solution, freshly prepared, and injected intraperitoneally to rats that had fasted for one night. In the nondiabetic group, the same volume of a 0.9% NaCl solution was injected intraperitoneally. Following alloxan administration, the pancreas secretes insulin at high levels and fatal hypoglycemia can occur. To prevent this adverse affect, 4–6 h after alloxan treatment, 5 mL of 20% glucose solution was injected intraperitoneally. Subsequently, 5% glucose solution was added to the drinking water of the rats for 24 h to prevent possible hypoglycemia. Fasting blood samples were collected 72 h later through the tail vein, and blood glucose levels were measured using an Accu-Check Active blood glucose monitor. At the end of the third day, animals having serum glucose levels higher than 200 mg/dL were considered diabetic and were included in the study. Body weight, blood glucose, and appetite were monitored 30 days after alloxan injection. Daily food intake of the rats was decreased and related weight loss was observed (). However, blood glucose levels were always greater than 200 mg/dL during the experiment.
TABLE 2. Differences between weights of the rats after waiting period
Ovariectomy surgery
During the acclimatization period, the rats were fed a standard commercial rat diet. One week before starting the experiment, four groups of animals, or 60 rats in total, underwent bilateral ovariectomy. For this procedure, the animals were anesthetized with 25 mg/kg sodium thiopental and injected intraperitoneally. A longitudinal incision (0.5–1 cm) was made in the midline area of the lower abdomen and the ovaries were removed.
Sepsis model
CLP model was applied in the rats as described by Wichterman, with minor modification.Citation21 Anesthesia was induced by intraperitoneal administration of sodium thiopental 25 mg/kg. After shaving the abdomen, the peritoneum was opened. Once the diaphragm exposed the abdominal organs, the cecum was isolated and ligated with a 3-0 silk ligature, just distally to the ileocecal valve. Two punctures were made through the cecum distally using a 12-gauge needle to the point of ligation, and the cecum was returned to the peritoneal cavity. The abdominal incision was then closed with a 4/0 sterile synthetic absorbable suture. A laparotomy was performed on animals in the sham-operated group and the cecum was manipulated, but not ligated or perforated. All animals were administered 2 mL/100 g body weight of normal saline subcutaneously at the time of surgery and also at 6 h postoperatively for fluid resuscitation. Postoperatively, the rats were deprived of food, but had free access to water for the next 20 h until they were killed.
All five groups of rats were killed 20 h later by an overdose of a general anesthetic (sodium thiopental, 50 mg/kg). The kidneys were rapidly removed from all rats and washed in ice-cold saline. They were labeled and stored at −80°C until analysis. At the end of the experiment, 10 rats remained in the OVX+CLP group, nine rats in the DM+OVX+CLP group, 15 rats in the sham-operated group, and 15 rats in the OVX group. Samples from dead animals were not processed for histopathology, serum examination, and biochemistry. Thus, nine randomized animals were selected from each group for further experiments.
Biochemical investigation of kidney tissues
After macroscopic analyses, superoxide dismutase (SOD), catalase (CAT), lipid peroxidation (LPO) activity, and glutathione (GSH) enzyme levels were determined in rat kidney tissues. To prepare the tissue homogenates, tissues were macerated with liquid nitrogen in a mortar. The ground tissues (0.5 g each) were subsequently treated with 4.5 mL of the appropriate buffer. The mixtures were homogenized on ice using an Ultra-Turrax Homogenizer for 15 min. The homogenates were filtered and centrifuged by a refrigerated centrifuge at 4°C. These supernatants were then used for the determination of enzymatic activities. All assays were performed at room temperature in triplicate.
SOD activity
Measurements were performed according to Sun et al.Citation22 SOD estimation was based on the generation of superoxide radicals produced by xanthine and xanthine oxidase, which react with nitro blue tetrazolium (NTB) to form formazan dye. SOD activity was then measured at 560 nm by the degree of inhibition of this reaction, and was expressed as millimole per minute per milligram of tissue (mmol/min/mg tissue).
CAT activity
Decomposition of H2O2in the presence of CAT was performed at 240 nm.Citation23 CAT activity was defined as the amount of enzyme required to decompose 1 nm of H2O2per minute at 25°C and pH 7.8. Results are expressed as millimole per minute per milligram of tissue (mmol/min/mg tissue).
Determination of LPO
LPO in tissue was determined by estimating the level of malondialdehyde (MDA) using the thiobarbituric acid test.Citation24 The rats' stomachs were promptly excised and rinsed with cold saline. To minimize the possibility of interference of hemoglobin with free radicals, any blood adhering to the mucosa was carefully removed. The corpus mucosa was scraped, weighed, and homogenized in 10 mL of 100 g/L KCl. The homogenate (0.5 mL) was added to a solution containing 0.2 mL of 80 g/L sodium laurylsulfate, 1.5 mL of 200 g/L acetic acid, 1.5 mL of 8 g/L 2-thiobarbiturate, and 0.3 mL distilled water. The mixture was incubated at 98°C for 1 h. Upon cooling, 5 mL of n-butanol : pyridine (15 : l) was added. The mixture was centrifuged for 30 min at 4000 rpm (2860 × g). The supernatant was measured at 532 nm and a standard curve was obtained using 1,1,3,3-tetramethoxypropane. The recovery was over 90%. The results were expressed as nanomole of MDA per gram of tissue (nmol MDA/g).
GSH determination
The amounts of GSH in the tissues were measured according to the method of Sedlak and Lindsay.Citation25 The mucosal surface of the stomach was collected by scraping and was then weighed and homogenized in 2 mL of 50 mM Tris-HCl buffer containing 20 mM EDTA and 0.2 M sucrose, pH 7.5. The homogenate was immediately precipitated with 0.1 mL of 25% trichloroacetic acid and the precipitate was removed after centrifugation at 4200 rpm (3150 × g) for 40 min at 4°C. The supernatant was used to determine GSH using 5,5′-dithiobis (2-nitrobenzoic acid). Absorbance was measured at 412 nm using a spectrophotometer. The results of the GSH levels in the tissues were expressed as nanomoles per milligram of tissue (nmol/mg tissue).
Determination of serum enzymes
The separated serums were used for the determination of blood urea nitrogen (BUN) and creatinine activities with an autoanalyzer (Cobas C-501, Roche Diagnostics, USA).
Histological procedures
Microscopy
The kidney samples for light microscopic examination were fixed in 10% formaldehyde, dehydrated in a graded alcohol series, and cleared in xylol. After dehydration, specimens were embedded in fresh paraffin (Agar, Istanbul, Turkey). Sections were cut using a microtome (Leica, Istanbul, Turkey). Each paraffin block was serially cut into 5-μm thickness. The sections were stained with hematoxylin–eosin (HE) for light microscopic examination.
Immunohistochemical staining of NF-κB
Paraffin-embedded kidney samples were used for NF-κB immunohistochemistry. Tissue sections of 5 μm were deparaffinized in xylene and rehydrated in ethanol followed by water and phosphate-buffered saline (PBS). Endogenous peroxidase was blocked by immersion in 3% hydrogen peroxide. The tissue sections were incubated with an NF-κB antibody (Dako, Istanbul, Turkey) at a concentration of 5 μg/mL for 1 h at room temperature. Control sections were incubated with PBS containing normal goat serum without a primary antibody. Immunostaining was detected with a streptavidin–biotin complex (SABC) kit (Dako, Istanbul, Turkey) and developed with diaminobenzidine tetrahydrochloride. The sections were counterstained with Mayer's hematoxylin followed by light microscopy.
Statistical analysis
Statistical analysis of oxidant and antioxidant enzymes was conducted using one-way analysis of variance (ANOVA) followed by Duncan's multiple range test (DMRT) using the SPSS software package, version 12.00, and were considered significant at p< 0.05. All results were expressed as mean ± SE for the nine rats in each group.
RESULTS
Mortality
In the ovariectomized and septic group, 5 out of the 15 rats (33%) died between 12 and 20 h after CLP-induced sepsis and in the diabetes + sepsis + ovariectomy group, 6 (6/15) or 40% of the rats died between 6 and 20 h. No mortality was observed in the control group or in the diabetes control group during CLP.
Biochemical results for oxidant and antioxidant levels of kidney tissue in rats
Effects of diabetes and sepsis on CAT and SOD enzyme activities and LPO and GSH levels in ovariectomized rat kidney tissue are demonstrated in . The CAT activity and LPO and GSH levels were increased, and SOD activity was decreased in the ovariectomized group when compared to the control groups (p< 0.05). Similarly, both sepsis and diabetes treatments exhibited an increase in the activity of CAT and in the amounts of LPO and GSH and demonstrated a decrease in SOD activity when compared to the ovariectomized group (p< 0.05). Ovariectomy significantly increased CAT activity in rat kidney tissue versus the control group. The enzyme activity also showed a significant increase in kidney tissues in diabetic, septic, and diabetic–septic ovariectomized rats. The highest activity was measured in diabetic ovariectomized rats. Ovariectomy, diabetes, and sepsis decreased SOD activity significantly. The lowest SOD activity was measured in the diabetic–septic ovariectomized rat group. Ovariectomy, sepsis, and diabetes increased both LPO and GSH levels as compared to rats in the control group. The lowest LPO and GSH levels were measured in the control group, whereas the greatest levels were determined in diabetic–septic ovariectomized rats.
TABLE 3. The effects of ovariectomy on total glutathione (GSH), lipid peroxidation levels, enzymatic activities of LPO, catalase (CAT), and superoxide dismutase (SOD) of sepsis, diabetes, and sepsis and diabetes on kidney tissues of rats
Effects of diabetes and/or sepsis in CLP-induced sepsis of rats on serum glucose, BUN, creatinine, and weight
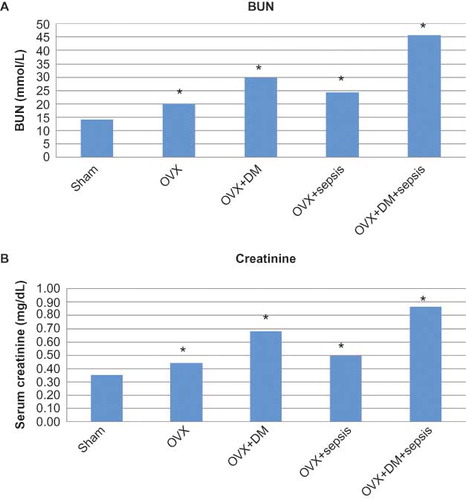
As illustrated in and , alloxan-treated animals increased blood glucose levels and reduced weight significantly when compared with the control group. The effects of sepsis and diabetes on the serum enzymes BUN and creatinine are shown in A and B. Alloxan administration significantly increased the activities of the serum enzymes when compared with the control (p< 0.01). The greatest production of BUN and creatinine serum levels was observed in the group that experienced diabetes + ovariectomy + CLP-induced sepsis (p< 0.01). Additionally, the sepsis + ovariectomy treated group significantly increased the activities of the serum enzymes when compared with the control (p< 0.01).
Histopathological results
Conventional light microscopic examination
The kidneys removed from control rats consisted of the cortex and the medulla. The renal cortex of the control rats was composed of glomeruli, blood vessels, tubules, and interstitium. When evaluating these renal specimens using light microscopy on HE-stained sections, the overall cellularity and the symmetry of the glomeruli were detected. Renal tubules formed as the proximal tubule and the distal tubule. The walls of all kinds of tubules were formed by continued cells. Among tubular structures, very little interstitium was present in the cortex (A and B).
FIGURE 3. Light microscopy of kidney in the control (A, B) and the ovariectomy group (C, D). gl, glomerulus; d, distal tubules; p, proximal tubules; t, collecting tubules; dye: hematoxylin–eosin; magnification bars: 40 μm.
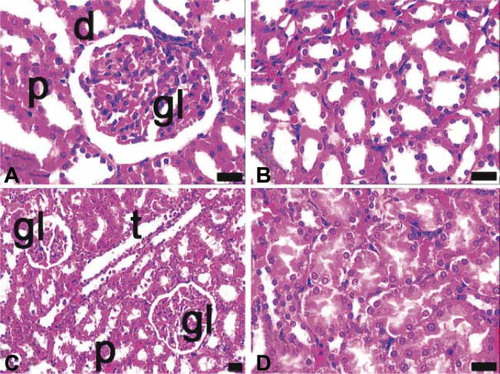
Light microscopic findings of kidney sections from ovariectomized, septic, and diabetic rats were summarized as follows. In the ovariectomized group, irregularity of tubules, especially proximal tubules were observed (C and D).
In the diabetic group, destruction in the proximal tubules was greater than in the ovariectomized group. Tubular integrity was lost and the apical portions of the proximal tubules were vascularized. Sclerotic glomeruli, hyaline accumulation, and mild inflammatory cell infiltration were observed in this group (A and B).
FIGURE 4. Light microscopy of kidney in the diabetes (A, B) and sepsis group (C, D). gl, glomerulus; p, proximal tubules; asterisk, reduced glomerulus; underlined asterisk, inflammatory cell infiltrations at nodular form; arrow, proximal tubules with apical degenerations; arrow with double head, inflammatory cell infiltrations at diffuse form; asterisk with white filling, proximal tubule with integrity loss; arrow head, eosinophilic accumulations in intertubular area; dye: hematoxylin–eosin; magnification bars: 40 μm.
In septic rats, faint apical degenerations in proximal tubules occurred, but they were not as prominent as in the diabetic rats. Additionally, inflammatory cell infiltration was observed in the perivascular region and glomerular capillaries were dilated (C and D).
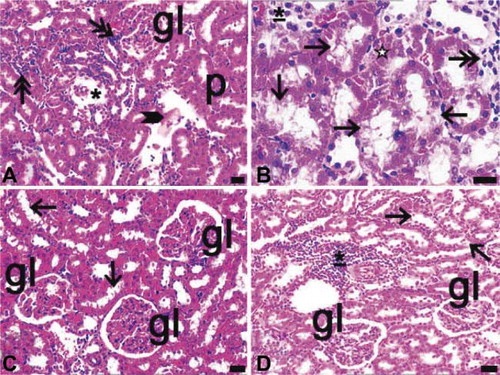
In ovariectomized and diabetic rats, the glomerular capillaries were dilated, both proximal and distal tubules degenerated, the structure of the proximal tubules was disrupted, and hydropic degeneration was observed in distal tubule cells (A and B).
FIGURE 5. Light microscopy of kidney in the ovariectomy and diabetes (A, B) and ovariectomy and sepsis (C, D), and diabetes and sepsis group (E, F). gl, glomerulus; asterisk, glomerulus with segmental necrosis; double asterisks, inflammatory cell infiltrations at diffuse form; thick arrow, inflammatory cell infiltrations at nodular form; thin arrow, cells of distal tubules with hydropic degenerations ; arrow with double head and inset of E, proximal tubules with apical degenerations, asterisk with white filling, glomeruli which their capillaries were congested arrowhead, erythrocytes in inter-tubular area; dye: hematoxylin–eosin; magnification bars: 40 μm.
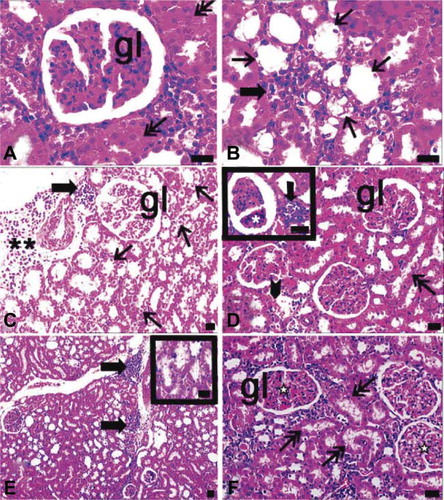
In ovariectomized and septic rats, subcapsular, localized, diffuse inflammatory cell infiltration was detected. Moreover, in the periarteriolar region, inflammatory cell infiltration was noted in the nodular form. Glomeruli in this group showed capillary dilation and segmental necrosis. Distal tubules exhibited hydropic degeneration and the apical surface of the proximal tubule was irregular. A few erythrocytes were present among the cortical tubules (C and D).
In both diabetic and septic rats, dilatation was observed especially in the branches of cortical vessels. Inflammatory cell infiltrations were nodular in form and some diffuse infiltration sites were defined at the periphery of glomeruli. In addition, capillary dilatation and mild congestion were defined in glomeruli (E and F).
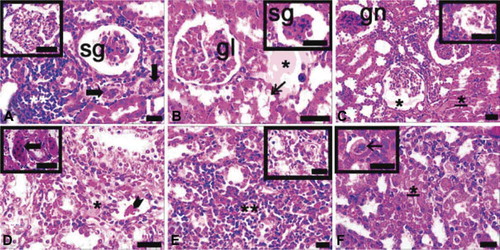
When sections of ovariectomized, septic, and diabetic rats were evaluated histologically, glomerular capillaries were dilated and sclerotic, or fully degenerated glomeruli were observed. Some glomeruli were infiltrated by inflammatory cells. In most areas, not only proximal but also distal tubules were degenerated and their integrities were lost. Proteinaceous or eosinophilic materials were accumulated in urinary spaces and between cortical tubules. Extramesangial cells were proliferated and multiple giant cells were found. In the cortex, nodular and diffuse inflammation sites were observed (A–F).
FIGURE 6. Light microscopy of kidney in the ovariectomy, sepsis and diabetes group (A–F). gl, glomerulus; sg, sclerotic glomeruli; gn, glomerulonephritis; asterisk, proteinous accumulations in inter-tubular area and urinary space; double asterisks, inflammatory cell infiltrations at diffuse form; underlined asterisk, proximal tubule with integrity loss; thick arrows, proliferating extra-mesangial cells in cortex; thin arrow, giant cells in cortex; inset of A, glomeruli with dilated capillary; inset of C, reduced glomerulus; inset of E, degenerated tubules; dye: hematoxylin–eosin; magnification bars: 40 μm.
Immunohistochemical results
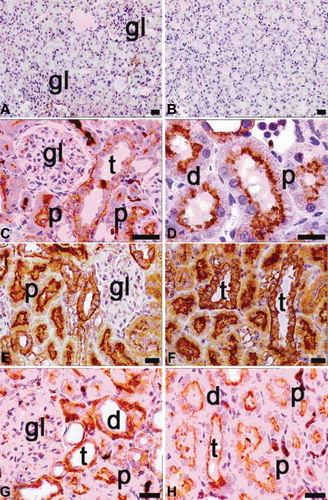
NF-κB activation and subsequent induction of expression of multiple proinflammatory molecules is considered to be central to the pathogenesis of renal inflammation. NF-κB phosphorylation is a prerequisite and a marker for NF-κB activation.Citation26 NF-κB activation was examined in vitroin kidneys obtained from ovariectomy, septic, and diabetic models or binary/triplicate combinations by using a specific kit for phosphorylated NF-κB p65 (p-p65). By immunohistochemistry, kidneys from control rats displayed the smallest positive staining for activated NF-κB, primarily located in the basal cytoplasm of tubular epithelial cells (A and B).
FIGURE 7. Immunohistochemical staining of NF-κB (p65) in tissue sections of control (A, B), ovariectomy (C, D), sepsis (E, F), and diabetes (G, H) groups. gl, glomerulus; d, distal tubules; p, proximal tubules; t, collecting tubules; positivity was seen in especially cytoplasmic pattern of tubular cells in ovariectomy (C, D), sepsis (E, F; the most expression of p65), and diabetes (G, H) groups; magnification bars: 30 μm.
Ovariectomized, septic, and diabetic rats, alone or in combination, demonstrated NF-κB activation (). The maximal p65 expression was detected in the ovariectomized, septic, and diabetic group (A–D). The p65 subunit of the NF-κB complex was sparsely expressed in the tubules of only ovariectomized (C and D) and only diabetic rats (G and H). Virtually no cells appeared in the glomerular or interstitial areas in these views. In septic rats, tubular p65 expression was also scant, but it was greater than in the only ovariectomized and in the only diabetic groups (E and F). When the effects of binary combinations of ovariectomy, sepsis, and diabetes on NF-κB activation were evaluated immunohistochemically, many p65-positive cells were observed, especially in the apical surface of tubular cells and in the cytoplasm and nuclei of extramesangial cells in ovariectomized and diabetic rats (A and B). The positivity of p65 was increased in kidneys of ovariectomized septic rats relative to those of ovariectomized diabetic rats (C and D). Nevertheless, p65-positive mesangial cells were observed in glomeruli of ovariectomized septic rats (C and D).
FIGURE 8. Immunohistochemical staining of NF-κB (p65) in tissue sections of ovariectomy–diabetes (A, B), ovariectomy–sepsis (C, D), and diabetes–sepsis (E, F) groups. gl, glomerulus; d, distal tubules; p, proximal tubules; t, collecting tubules; arrows indicate extramesangial cells with p65 positive nuclei and cytoplasm; positivity was seen in especially cytoplasmic pattern of tubular cells, both cytoplasmic and nuclear pattern of extramesangial cells and rarely in glomeruli. The most expression of p65 was found in diabetes–sepsis group; magnification bars: 30 μm.
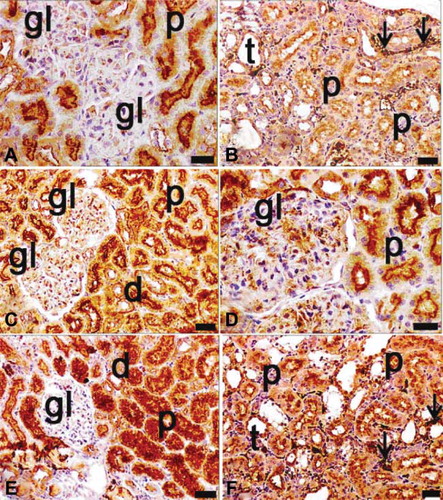
FIGURE 9. Immunohistochemical staining of NF-κ B (p65) in tissue sections of ovariectomy–diabetes and sepsis group (A–D). gl, glomerulus; d, distal tubules; p, proximal tubules; t, collecting tubules; arrows indicate extramesangial cells with p65 positive nuclei and cytoplasm; positivity was seen in especially cytoplasmic pattern of tubular cells, both cytoplasmic and nuclear pattern of extramesangial cells and commonly in glomeruli. The highest expression of p65 was found in this group among the all groups; magnification bars: 30 μm.
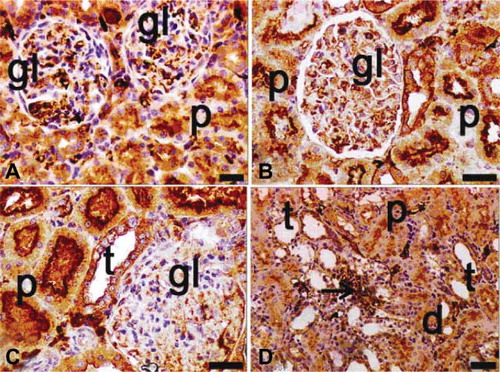
Among the binary groups of treatments, the greatest NF-κB expression was found in tubule and extramesangial cells of the septic and diabetic group (E and F). Finally, in the ovariectomized, septic, and diabetic group, NF-κB activation was significantly greater than in other groups (A–D). Positivity was detected in glomeruli, tubules, and extramesangial cells (A–D).
DISCUSSION
In this study, the rat intra-abdominal sepsis model was used to understand the role of sustained hyperglycemia and ovariectomy, either separately or concomitantly, on the response of NF-κB activation and oxidative response on kidney tissues in rats. Sepsis is a leading cause of renal failure.Citation27,Citation28 To explore this hypothesis, CLP was induced in a rat model of alloxan-induced diabetes in ovariectomized rats. This sepsis model was induced by ligation and perforation, which mimicked the clinical situation of bowel perforation and bacterial infection in humans.Citation21,Citation29 The hyperdynamic phase was demonstrated to persist from 2 to 10 h and the hypodynamic phase occurred at 16 h and lasted until 20 h after CLP.Citation30
In this study, the late phase of sepsis was accepted to be equal to the experimental model at the 20th hour. The changes in serum BUN and creatinine levels were examined following CLP. The study indicated that in the CLP-induced sepsis + ovariectomy + diabetes group, serum BUN and creatinine levels were significantly increased when compared to the control, ovariectomy + diabetes and ovariectomy only groups. Diabetes + ovariectomy caused a significant increase in serum levels of serum BUN and creatinine levels in comparison to the control group. The results of this study clearly indicated that diabetes and sepsis markedly increased both the plasma BUN and creatinine levels.
In female rats, ovariectomy is a commonly used animal model for examining the effects of estrogen insufficiency and metabolic consequences that artificially induce a marked reduction in endogenous estrogen concentrations.Citation31 Additionally, many of the problems associated with DM involve inflammation, sepsis, tissue damage, and oxidative stress. Hyperglycemia plays a significant role in the development of postoperative infections.Citation32 Furthermore, Leonidou et al.Citation33 demonstrated that hyperglycemia due to stress is associated with increased cytokine production following sepsis. In this study, it was demonstrated that DM, combined with ovariectomy, augmented the levels of oxidant in kidney tissue. They also exacerbate oxidative injury, as assessed by increased levels of LPO and GSH in a CLP-induced sepsis model. Rat exposure to CLP-increased LPO levels in the kidney tissue thereby indicated the infiltration of polymorphonuclear neutrophils and the development of oxidative tissue injury.
LPO, as a marker of oxidative damage, can cause changes in membrane fluidity and permeability and thus increase the rate of protein degradation, which will eventually lead to cell lysis.Citation34 Increased concentrations of tissue LPO levels are found in sepsis induced by cecal ligation and perforation in rats and humans.Citation35,Citation36
In this study, the LPO levels significantly increased in kidney tissue. The increases were greater after diabetes and ovariectomy in septic rats. This observation is in agreement with previous studies, which showed increased levels of lipid products as a result of oxidative stress.Citation37,Citation38
Furthermore, it was found that ovariectomy and diabetes increased LPO levels, which suggested that by abolishing cellular integrity, ovariectomy and diabetes could augment sepsis-induced organ damage.
Various studies have focused on ovariectomy-induced LPO in various tissues, such as the liver and kidneys, which caused a decline in ovarian hormones. In contrast, female gonadal hormones protect against oxidative stress by activating the antioxidant system.Citation39,Citation40
GSH is an important constituent of the intracellular protective role against oxidative stress. However, reduced GSH, as the primary component of the endogenous nonprotein sulfhydryl pool, is bonded to a variety of electrophilic radicals.Citation41 Recent studies have shown that serum GSH decreased in adults and children with septic shock.Citation42 GSH depletion, which is often associated with sepsis, was suggested to be detrimental, impairing the host response to infection.Citation43
In this study, the levels of GSH were significantly increased in the kidney tissues studied. The increases were greater in treatments having diabetes in addition to ovariectomy in septic rats. We suggested that the increase in GSH level may be a response of kidney tissue as augmentation of antioxidant defense mechanisms against increased oxidative stress.
In this study, the levels of CAT and SOD were significantly increased in the kidney tissues studied. Souza et al.Citation44 showed an increase of macrophage CAT activity, probably to prevent oxidative damage secondary to CLP in rats. In contradistinction to SOD activities in kidney tissues, this study showed that the SOD activities were significantly decreased in kidney tissues studied. The decrease was greater after diabetes in addition to ovariectomy in septic rats.
In physiological conditions, small amounts of generated ROSs are neutralized by antioxidative factors. When ROS generation increases, antioxidant defense systems fail and damage occurs. This may explain why the GSH levels and SOD activities decreased in the kidney tissue. The decrease in SOD and increase in the GSH and LPO level provoked the increase of markers of kidney damage, such as BUN and creatinine. This point of view is based at least partially in the important role of the antioxidant enzymes (SOD, CAT, and GSH) on the molecular control of ROSs and the prevention of their deleterious effects.Citation45 Potential effects of diabetes and ovariectomy on CLP-induced sepsis have also been detected in the histological and immunohistochemical findings. CLP was determined to produce evident injury in the kidneys of rats when compared with the control group, whereas the severity of the injury was greater in the diabetes + ovariectomy + CLP group when compared to the CLP group. In immunohistochemical staining, we determined that the CLP operation increased NF-κB activation. However, in CLP-operated and alloxan-administered rats, NF-κB positivity was increased relative to the CLP group. Although diabetes and ovariectomy effectively increased NF-κB activation in our models, the effects of these treatments potentially worsened with sepsis. The effects of inhibiting a mediator as central to the inflammatory response as NF-κB during sepsis and septic shock may be complex and difficult to predict. In the ovariectomized, septic, and diabetic group, NF-κB activation was significantly greater than in other groups.
NF-κB activation and the subsequent induction of the expression of multiple proinflammatory molecules are considered to be central to the pathogenesis of inflammation.Citation26 NF-κB activation and subsequent induction of expression of multiple proinflammatory molecules is considered to be central to the pathogenesis of renal inflammation. NF-κB phosphorylation is a prerequisite and a marker for NF-κB activation.Citation26 NF-κB, a transcription factor which plays a central regulatory role in inflammatory events such as sepsis, is now a leading target for anti-inflammatory agents developed to ameliorate this lethal syndrome.Citation46,Citation47 Accordingly, inhibition of the NF-κB signaling pathway is believed to be beneficial in the setting of septic shock.Citation48,Citation49.
The results of this study demonstrated that in rat groups with increased NF-κB activation in kidney tissues, in which sections of ovariectomized, septic, and diabetic rats were evaluated histologically, glomerular capillaries were dilated, sclerotic, or fully degenerated glomeruli were observed. Some glomeruli were infiltrated by inflammatory cells. In most areas, not only proximal but also distal tubules were degenerated and their integrity was lost. The treatment involving diabetes + ovariectomy with CLP challenge was associated with early increases in both NF-κB expression and histopathological results. The in vivofindings from this study were similar to other preclinical investigations showing that sepsis challenge produces increased tissue NF-κB activation.Citation50
Based on this preliminary investigation, the authors conclude that diabetic and ovariectomized rats with CLP-induced sepsis demonstrated increased serum BUN and creatinine levels. These levels correlated positively with tissue oxidant levels, such as LPO and GSH levels, which resulted in the exacerbation of oxidative kidney injury. The kidney tissue was most affected by diabetes and ovariectomy under sepsis conditions. Hyperglycemia and ovariectomy (postmenopausal period) severely increased serum BUN and creatinine and oxidant levels and NF-κB activity during the stages of our sepsis model. Ovariectomy leading to estrogen deficiency resulted in general changes in metabolism, which are observed in the kidney under sepsis conditions.
Acknowledgments
This research was conducted in the Laboratory of Pharmacology at Ataturk University, School of Medicine, Erzurum, Turkey, and the Laboratory of Histology and Embryology, School of Medicine at Ataturk University, Erzurum, Turkey. None of the authors has a commercial interest, financial interest, and/or other relationship with manufacturers of pharmaceuticals, laboratory supplies, and/or medical devices or with Commercial providers of medically related services.
REFERENCES
- Angus DC, Wax RS. Epidemiology of sepsis: An update. Crit Care Med. 2001;29:109–116.
- Annane D, Aegerter P, Jars-Guincestre MC, Current epidemiology of septic shock: The CUB-Rea Network. Am J Respir Crit Care Med. 2003;168:165–172.
- Doi K, Leelahavanichkul A, Hu X, Pre-existing renal disease promotes sepsis-induced acute kidney injury and worsens outcome. Kidney Int. 2008;74:991–993.
- Russell JA, Singer J, Bernard GR, Changing pattern of organ dysfunction in early human sepsis is related to mortality. Crit Care Med. 2000;28:3405–3411.
- Higuchi Y, Kawakami S, Hashida M. Development of cell-selective targeting systems of NFkappaB decoy for inflammation therapy. Yakugaku Zasshi. 2008;128:209–218.
- Tergaonkar V. NFkappaB pathway: A good signaling paradigm and therapeutic target. Int J Biochem Cell Biol. 2006;38:1647–1653.
- Kaya H, Sezik M, Ozkaya O, Lipid peroxidation at various estradiol concentrations in human circulation during ovarian stimulation with exogenous gonadotropins. Horm Metab Res. 2004;36:693–695.
- Prediger ME, Siqueira IR, Gamaro GD, Protective effect of pregnanolone against lipoperoxidation and free radicals generation induced in hypothalamus of ovariectomized rats submitted to CO2 exposure. Pharmacol Biochem Behav. 2004;78:191–197.
- Berco M, Bhavnani BR. Differential neuroprotective effects of equine estrogens against oxidized low density lipoprotein-induced neuronal cell death. J Soc Gynecol Investig. 2001;8:245–254.
- Herrington DM, Klein KP. Effects of SERMs on important indicators of cardiovascular health: Lipoproteins, hemostatic factors, and endothelial function. Womens Health Issues. 2001;11:95–102.
- Bernardi F, Pluchino N, Stomati M, CNS: Sex steroids and SERMs. Ann N Y Acad Sci. 2003;997:378–388.
- Ruiz-Larrea MB, Martín C, Martínez R, Antioxidant activities of estrogens against aqueous and lipophilic radicals; differences between phenol and catechol estrogens. Chem Phys Lipids. 2000;105:179–188.
- Galli F, Piroddi M, Annetti C, Oxidative stress and reactive oxygen species. Contrib Nephrol. 2005;149:240–260.
- Wagner JD, Thomas MJ, Williams JK, Insulin sensitivity and cardiovascular risk factors in ovariectomized monkeys with estradiol alone or combined with nomegestrol acetate. J Clin Endocrinol Metab. 1998;83:896–901.
- Kumagai S, Holmang A, Bjorntorp P. The effects of oestrogen and progesterone on insulin sensitivity in female rats. Acta Physiol Scand. 1993;149:91–97.
- Szekacs B, Vajo Z, Varbiro S, Postmenopausal hormone replacement improves proteinuria and impaired creatinine clearance in type 2 diabetes mellitus and hypertension. BJOG. 2000;107:1017–1021.
- Nuutila P, Knuuti MJ, Maki M, Gender and insulin sensitivity in the heart and in skeletal muscles. Studies using positron emission tomography. Diabetes. 1995;44:31–36.
- Donahue RP, Bean JA, Donahue RA, Insulin response in a triethnic population: Effects of sex, ethnic origin, and body fat. Miami Community Health Study. Diabetes Care. 1997;20:1670–1676.
- Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res. 2001;50:536–546.
- Gupta SS. Prospects and perspectives of natural plant products in medicine. Indian J Pharmacol. 1994;26:1–12.
- Wichterman KA, Baue AE, Chaudry IH. Sepsis and septic shock: A review of laboratory models and a proposal. J Surg Res. 1980;29:189–201.
- Sun Y, Oberley LW, Li Y. A simple method for clinical assay of superoxide dismutase. Clin Chem. 1988;34:497–500.
- Aebi H. Catalase. Medhods Enzymol. 1984;105:121–126.
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxide in animal tissues by thiobarbutiric acid reaction. Anal Biochem. 1979;95:351–358.
- Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryls groups in tissue with Ellman's reagent. Anal Biochem. 1968;25:192–205.
- Sakurai H, Chiba H, Miyoshi H, IκB kinases phosphorylate NF-kappaB p65 subunit on serine 536 in the transactivation domain. J Biol Chem. 1999;274:30353–30356.
- Uchino S, Kellum JA, Bellomo R, Acute renal failure in critically ill patients: A multinational, multicenter study. JAMA. 2005;294:813–818.
- Schor N. Acute renal failure and the sepsis syndrome. Kidney Int. 2002;61:764–776.
- Fink MP. Animal models of sepsis and its complications. Kidney Int. 2008;74:1017–1025.
- Remick DG, Newcomb DE, Bolgos GL, Comparison of the mortality and inflammatory response of two models of sepsis: Lipopolysaccharide vs. cecal ligation and puncture. Shock. 2000;13:110–116.
- Turner RT, Maran A, Lotinun S, Animal models for osteoporosis. Rev Endocr Metab Disord. 2001;2:117–127.
- Pomposelli JJ, Baxter JK, 3rd, Babineau TJ, Early postoperative glucose control predicts nosocomial infection rate in diabetic patients. JPEN J Parenter Enteral Nutr. 1998;22:77–81.
- Leonidou L, Mouzaki A, Michalaki M, Cytokine production and hospital mortality in patients with sepsis-induced stress hyperglycemia. J Infect. 2007;55:340–346.
- García JJ, Reiter RJ, Guerrero JM, Melatonin prevents changes in microsomal membrane fluidity during induced lipid peroxidation. FEBS Lett. 1997;408:297–300.
- Ortolani O, Conti A, De Gaudio AR, The effect of glutathione and N-acetylcysteine on lipid peroxidative damage in patients with early septic shock. Am J Respir Crit Care Med. 2000;16:1907–1911.
- Kharb S, Singh V, Ghalaut PS, Role of oxygen free radicals in shock. J Assoc Physicians India. 2000;48:956–957.
- Supinski G, Stofan D, Callahan LA, Peroxynitrite induces contractile dysfunction and lipid peroxidation in the diaphragm. J Appl Physiol. 1999;87:783–791.
- Sener G, Sehirli AO, Keyer-Uysal M, The protective effect of melatonin on renal ischemiareperfusion in rat. J Pineal Res. 2002;32:120–126.
- Oge A, Sezer ED, Ozgonul M, The effects of estrogen and raloxifene treatment on the antioxidant enzymes and nitrite-nitrate levels in brain cortex of ovariectomized rats. Neurosci Lett. 2003;338:217–220.
- Oztekin E, Tiftik AM, Baltaci AK, Lipid peroxidation in liver tissue of ovariectomized and pinealectomized rats: Effect of estradiol and progesterone supplementation. Cell Biochem Funct. 2006;25:401–405.
- Ross D. Glutathione, free radicals and chemotherapeutic agents. Pharmacol Ther. 1988;37:231–249.
- Lyons J, Rauh-Pfeiffer A, Ming-Yu Y, Cysteine metabolism and whole blood glutathione synthesis in septic pediatric patients. Crit Care Med. 2001;29:870–877.
- Villa P, Saccani A, Sica A. Glutathione protects mice from lethal sepsis by limiting inflammation and potentiating host defense. J Infect Dis. 2002;185:1115–1120.
- de Souza LF, Ritter C, Pens Gelain D et al. Mitochondrial superoxide production is related to the control of cytokine release from peritoneal macrophage after antioxidant treatment in septic rats. J Surg Res. 2007;141:252–256.
- Matés JM. Effects of antioxidant enzymes in the molecular control of reactive oxygen species in toxicology. Biog Amines. 2000;16:53–62.
- Baldwin Jr AS. The NF-kappa B and I kappa B proteins: New discoveries and insights. Annu Rev Immunol. 1996;14:649–683.
- Cadirci E, Altunkaynak BZ, Halici Z, Alpha-lipoic acid as a potential target for the treatment of lung injury caused by cecal ligation and puncture-induced sepsis model in rats. Shock. 9 October 2009. DOI: 10.1097/SHK.0b013e3181c3cf0e..
- Hall G, Singh IS, Hester L, Inhibitor-kappaB kinase-beta regulates LPS-induced TNF-alpha production in cardiac myocytes through modulation of NF-kappaB p65 subunit phosphorylation. Am J Physiol Heart Circ Physiol. 2005;289:H2103–H2111.
- Haudek SB, Spencer E, Bryant DD, Overexpression of cardiac I-kappaBalpha prevents endotoxin-induced myocardial dysfunction. Am J Physiol Heart Circ Physiol. 2001;280:H962–H968.
- Blackwell TS, Yull FE, Chen CL, Multiorgan nuclear factor kappa B activation in a transgenic mouse model of systemic inflammation. Am J Respir Crit Care Med. 2000;162:1095–1101.