Abstract
Background: The kidney is a common target in multiple organ dysfunction syndrome (MODS). The aim of this study is to determine the role of intestinal lymphatic pathway on renal injury in hemorrhagic shock rats. Methods: Wistar rats were divided into sham, shock, and ligation groups. The hemorrhagic shock model was induced in the shock and ligation groups. After resuscitation, the mesenteric lymph ducts were ligated in the ligation group. Blood from the carotid artery was taken to determine renal functional indices. The kidneys were used to observe histomorphological changes at 6 h after resuscitation. In addition, kidney homogenate was used to determine malondialdehyde (MDA), superoxide dismutase (SOD), tumor necrosis factor alpha (TNFα), interleukin-6 (IL-6), and myeloperoxidase (MPO) levels at 90 min after shock and 0, 1, 3, 6, 12, and 24 h after resuscitation. And the survival rate of 24 h was recorded. Results: The survival rate in shock group was obviously lower than sham and ligation groups. The urea and creatinine contents in the serum of shock and ligation groups were significantly higher than the sham group; the indices in the ligation group were significantly lower than the shock group. Histological studies showed various degrees of renal injury in the shock and ligation groups with a lesser severity in the ligation group. MDA, TNFα, IL-6, and MPO in renal homogenate of the shock group were raised, and the activity of SOD was lower in comparison to the sham group. Further, MDA, TNFα, IL-6, and MPO in renal homogenate of the ligation group at 6, 12, and 24 h were lower, and the SOD activity was higher than that of the shock group at the same time points. Conclusion: The mesenteric lymph duct ligation could be used to attenuate renal injury in shock rats. Its mechanism might be related to reducing the polymorph nuclear (PMN) and decreasing inflammatory mediator and free radical.
INTRODUCTION
The multiple organ dysfunction syndrome (MODS) can be defined as the development of potentially reversible physiologic derangement involving two or more organ systems not involved in the disorder that resulted in ICU admission and arising in the wake of a potentially life-threatening physiologic insult.Citation1 Medically, MODS is of importance because it is the leading cause of death in surgical patients and in trauma patients who survive the first 24 h.Citation2 MODS is caused by several factors including shock, infection, and wounds. Among the many target organs, the kidney is one of the common organs that are affected.
Mesenteric lymphatic duct ligation has played an important role in the attenuation of MODS.Citation3 Studies within the microcirculation suggests that modulating the microcirculatory pathway may play a role in treating patients suffering from shock as well as preventing MODS. Further, it also leads to a gradual decrease in death for people suffering from renal insufficiency. Patients with renal insufficiency have increased free radical release and are subjected to the waterfalls effect of inflammatory mediators caused by the kidney injury. Stopping the mesenteric lymph fluid reflux can ease the injury of kidney, lung, liver, and heart in the MODS rats subject to two-hit of hemorrhage and lipopolysaccharide (LPS) and decrease the death rate.Citation4,Citation5 Hence, the intestinal lymph pathway plays an important role in multiple organ injury.
The aim of this study is to observe the effect of mesenteric lymph duct ligation on renal function and histomorphology in severe hemorrhagic shock rats and investigate its mechanism from free radical and inflammatory mediator in this study.
METHODS
Animals
One hundred and eight healthy and SPF Wistar rats (supplied by the Chinese Academy of Medical Sciences Animal Breeding Center) weighing 220–300 g were used in this study. Animals were maintained under barrier-sustained conditions with 12:12-hour light–dark cycles and free access to standard laboratory food and water. The research protocol was carried out after a minimal acclimatization period of 5 days, complied with the Guideline for the Care and Use of Laboratory Animals (NIH Publication, 1996), and was approved by the Institutional Animal Use and Care Committee of the Medical School, Hebei North University. The rats were forbidden to eat 12 h before experiment, but were allowed free drinking.
Grouping
Seventy-eight rats were divided equally into three groups: sham group, shock group (at 90 min after shock, and at 0, 1, 3, 6, 12, 24 h, etc., after transfusion and resuscitation all n = 6), ligation group (at 1, 3, 6, 12, 24 h, etc., after transfusion and resuscitation all n = 6). And the other 30 rats were used to record the survival rate of 24 h (in sham, shock, ligation groups all n = 10).
Hemorrhagic shock model duplicating
Rats were generally anesthetized with pentobarbital sodium (50 mg/kg) by intramuscular injection. Under sterile conditions, a tube was inserted into the right common artery and left jugular vein for leasing blood and transfusion and 0.5% heparin was injected into the vein (1 mL/kg, 700 U/mL) for whole body anticoagulant. After 10 min of stabilization, blood (one fifth of body volume and one thirteenth of avoirdupois) was drawn by the automatic withdrawal-infusion machine (type ZCZ-50, Zhejiang, China), the whole course was completed in 3 min, and the blood was reserved for refusing usage. Aseptic Green-abdominal fluid bottle was taken as lasson' bottle; the liquid level was adjusted to adjunctive height to maintain a low pressure (40 mmHg) for 90 min. Then the duplication of serious hemorrhagic shock model was completed. For the shock and ligation groups, the leased blood (the amount was the whole blood) and the Ringer's solution were reperfused in 20 min. Rats in the ligation group received abdominal operations after transfusion and resuscitation to ligate the mesenteric lymph duct, whereas for the rats in shock group, only a cotton below mesenteric lymph duct was threaded. The rats in sham group were only anesthetized and received neck and abdominal operation, and these animals were not subjected to blood loss, transfusion, and resuscitation.
Sample collecting
All rats in each subgroup were killed at different time points in anesthesia. Fixed kidney tissue was mixed with nine times 4°C normal saline. FJ200-type high-speed dispersion machine (China, Shanghai) was used to prepare 10% renal homogenate at low temperatures, by centrifuging for 10 min at the rate of 2500 rpm. The supernatant was stored at −80°C in refrigerator (Thermo Electron, MA, USA).
In addition, rats in all three groups had cervical operation 6 h after resuscitation; blood sample was withdrawn from the carotid artery catheter and centrifuged at 2500 rpm for 10 min (centrifugal radius 8 cm), and the serum was stored at −80°C for measuring the renal biochemical indices. The remaining renal tissue was harvested in a fixed position for kidney microscopic sections.
Survival rate of 24 h observation
The other 30 rats were divided equally into three groups: sham, shock, and ligation groups. According to above methods, all rats were anesthetized and received neck and abdominal operation, and the shock group and ligation group rats were subjected to blood loss, transfusion, and resuscitation. After 24 h, the survival rates were recorded.
Test of renal function
The urea and creatinine contents in serum were measured by an automatic biochemical analyzer (Aeroset, Abbott, Chicago, IL, USA and the kit from Randox Laboratories Ltd. Shanghai, China).
Morphological examination of kidney
After killing the rats, renal tissue was obtained in a fixed orientation and fixed in neutral buffered formalin to process into paraffin blocks which were sectioned at 5 μm. After hematoxylin and eosin (HE) staining, morphological alterations were examined by light microscopy (BH-2; Olympus, Tokyo, Japan) and pictures were taken using digital camera (4500; Nikon, Tokyo, Japan) from 10 randomly chosen areas per sample. All morphological examinations were done by a forensic pathologist without prior knowledge of experimental conditions.
Assay of MDA and SOD in renal homogenate
The concentrations of MDA and SOD in renal homogenate were determined by modified thiobarbituric acid (TBA) microdetermination and xanthinoxidase method (Jiancheng Biotechnology Research Institute, Nanjing, China). The homogenate protein was tested by Coomassie brilliant blue method.
Assay of TNFα and IL-6 in renal homogenate
The contents of tumor necrosis factor alpha (TNFα) and interleukin-6 (IL-6) in renal homogenate were measured by enzyme-linked immunoadsorbent assay (ELISA) method (Bosde Biotechnology Limited Company, Wuhan, China) according to the vendor's recommendation. SPSS 11.5 statistical software was used to draw the standard curve and the sample was computed.
Assay of MPO in renal homogenate
MPO activity of renal homogenate was measured by hydrogen peroxide method (tissue protein of each gram was decomposed 1 μmol as an enzyme activity unit in 37°C H2O2 reaction systems, and the kit was bought from Jiancheng Biotechnology Research Institute), which was operated strictly according to the kit instructions.
Statistical analysis
All data were presented as mean ± SD. The statistical analysis was performed by SPSS software 11.5. One-way analysis of variance (ANOVA) was used between groups and paired t-test was used within group and χ2 test was used in the survival rate comparing between groups, and exact probability was used within group. p-value < 0.05 was considered to be statistically significant.
RESULTS
Effect of mesenteric lymph duct ligation on survival rate of 24 h in hemorrhagic shock rats
There were six rats alive in shock group (6/10, 60%) and nine rats alive in ligation group (9/10, 90%), and the rats in sham group were all alive (10/10, 100%). There was significance in three groups (χ2 = 6.240, p = 0.044, < 0.05); and there was no statistically significant differences between sham group and ligation group (p = 0.999); the survival rate of the two groups were markedly higher than the shock group (p = 0.031, <0.05).
Effect of mesenteric lymph duct ligation on renal function indices in hemorrhagic shock rats
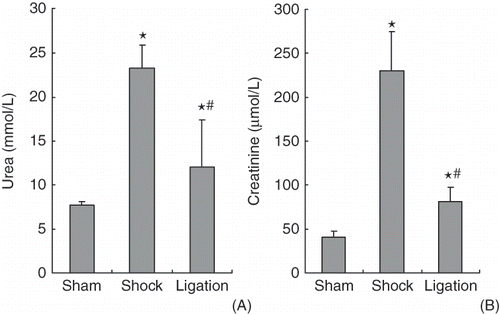
At 6 h after transfusion and resuscitation, urea and creatinine in serum of rats in shock and ligation groups were significantly higher than that of sham group rats, Interestingly, the urea and the creatinine in ligation group rats were lower than that of shock group rats (p < 0.01) (see ).
Effect of mesenteric lymph duct ligation on pathomorphology of kidney in hemorrhagic shock rats
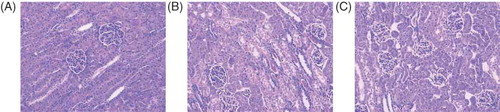
Rats in sham group have a normal architecture in renal glomerulus and tubules. Further, the proximal and distal convoluted tubules were clear and distinctive (). Rats in shock group demonstrated fibrinoid necrosis of glomerular capillary with proteinosis in renal capsule. Epithelial cells necrosis and protein casts in the tubules were also observed (). However, ligation of mesenteric lymphatic alleviates epithelial necrosis and protein casts of tubules; however, the fibrinoid necrosis of glomerular capillary had no significant changes ().
Effect of mesenteric lymph duct ligation on MDA content and SOD activity of kidney in hemorrhagic shock rats
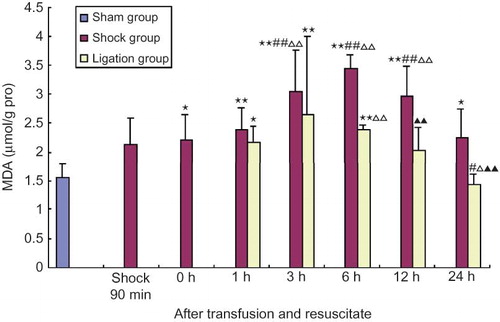
The content of MDA in renal homogenate from the shock group at 0, 1, 3, 6, 12, and 24 h after transfusion and resuscitation was higher than that of the sham group. Further, at 3, 6, and 12 h, the MDA levels were higher than that of 90 min after shock and 0 h after resuscitation (p < 0.01, < 0.05). However, in the ligation group, the renal homogenate at 1, 3, and 6 h after transfusion and resuscitation was higher than that in sham group, but the renal homogenate of ligation group at 24 h after resuscitation was lower than that of 90 min after shock and at 0 h after resuscitation and that at 0, 12, and 24 h after resuscitation was significantly lower than that in shock group at the same points (p < 0.01, < 0.05) (see ).
FIGURE 3. Effect of mesenteric lymph duct ligation on MDA content of kidney in hemorrhagic shock rats (mean ± SD, n = 6).
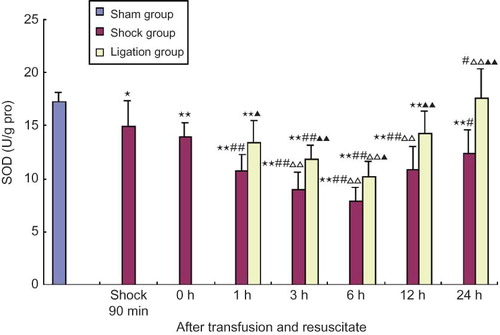
SOD activity in renal homogenate of rats in the shock group at 90 min after shock and the different points after transfusion and resuscitation was significantly lower than that in sham group, and that at all time points (1–24 h) after transfusion and resuscitation was lower than that of 90 min after shock. Further, SOD levels in rats from the shock group 3, 6, and 12 h after transfusion and resuscitation were significantly lower than that of shock resuscitation at 0 h (p < 0.01, < 0.05). In the ligation group, the activities of SOD in renal homogenate at 1, 3, 6, and 12 h after transfusion and resuscitation were lower than that in sham group, and they were obviously higher than that in shock group at the same time, respectively; at 3 and 6 h they were lower than that at 90 min after shock, and at 6 h they were lower than that at shock resuscitation at 0 h, and yet at 24 h SOD activity was obviously higher than that in shock group after shock resuscitation at 0 h (p < 0.01,< 0.05) (see ).
FIGURE 4. Effect of mesenteric lymph duct ligation on SOD activity of kidney in hemorrhagic shock rats (mean ± SD, n = 6).
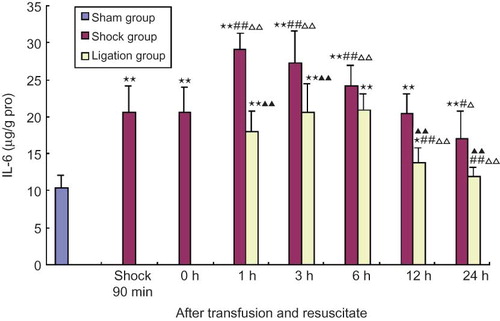
Effect of mesenteric lymph duct ligation on TNFα and IL-6 of renal homogenate in hemorrhagic shock rats
The contents of TNFα and IL-6 in renal homogenate of shock group at 90 min after shock and 0, 1, 3, 6, 12, 24 h after resuscitation were all significantly higher than that of sham group, and that at 1, 3, 6 h after resuscitation were significantly higher than 90 min after shock and after shock resuscitation at 0 h; IL-6 content in renal homogenate of shock group after resuscitation at 12 h was lower than that at 90 min after shock and after resuscitation at 0 h (p < 0.01, < 0.05). In ligation group, the contents of TNFα in renal homogenate at 1, 3, 6 h and IL-6 at 1, 3, 6, 12 h after transfusion and resuscitation were significantly higher than that in sham group; the contents of TNFα and IL-6 at 12 and 24 h after resuscitation were significantly lower than at 0 h after resuscitation and the content of IL-6 was obviously lower than that of 90 min after shock; the contents of TNFα at any point and IL-6 at 1, 3, 12, 24 h were all obviously lower than that in shock group at the same points (p < 0.01, < 0.05) (see and ).
FIGURE 5. Effect of mesenteric lymph duct ligation on TNFα content of kidney in hemorrhagic shock rats (mean ± SD, n = 6).
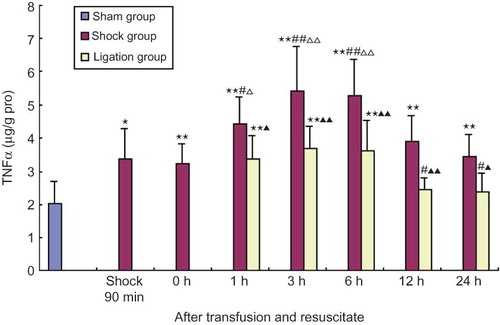
FIGURE 6. Effect of mesenteric lymph duct ligation on IL-6 content of kidney in hemorrhagic shock rats (mean ± SD, n = 6).
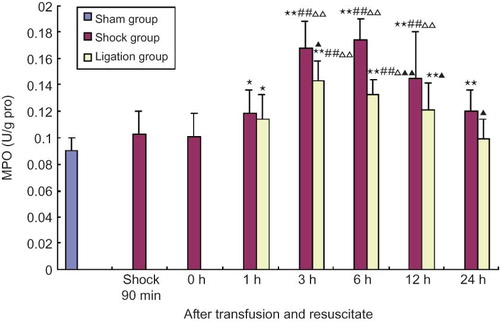
Effect of mesenteric lymph duct ligation on MPO activity of renal homogenate in hemorrhagic shock rats
The MPO activities in renal homogenate of shock group at 1, 3, 6, 12, 24 h after transfusion and resuscitation were obviously higher than that in sham group, and that at 3, 6, 12 h after transfusion and resuscitation were obviously higher than that of 90 min after shock and after transfusion and resuscitation at 0 h (p < 0.01, < 0.05). However, the MPO activities in renal homogenate of ligation group at 1, 3, 6, 12 h after transfusion and resuscitation were higher than that in sham group, that of 3 and 6 h after transfusion and resuscitation were significantly higher than that of 90 min after shock and 0 h after shock resuscitation, and that of 3, 6, 12, 24 h were all significantly lower than that in shock group at the same time points (see ).
FIGURE 7. Effect of mesenteric lymph duct ligation on MPO activity of kidney in hemorrhagic shock rats (mean ± SD, n = 6).
DISCUSSION
Gastrointestinal Infections, induced by bacteria/endotoxin translocation (BET) resulting from intestine barrier dysfunction, is an important connection in organ dysfunction induced by shock promoting the occurrence of MODS and systemic inflammatory response syndrome (SIRS). Further, it is also a significant factor in the occurrence of sepsis in severe patients who have no explicit infection.Citation6 Researches focusing on portal vein pathways of BET show that bacteria and toxin are not found in wound shock animal model or in blood from patients' portal vein,Citation3,Citation7 and shock plasma of portal vein had a light harm effect on vascular endothelial cell (VEC).Citation3,Citation8 These studies suggest that the portal vein pathway itself has limitations in causing organic injury reduced by shock. This study aims to explore the effect of mesenteric lymph duct ligation on renal insufficiency in shock rats by cutting off the intestine lymph return pathway of BET. The goal is to emphasize the importance of kidney damage in the occurrence of MODS induced by shock. Our study showed that mesenteric lymph duct ligation could lessen the degree of renal injury in hemorrhagic shock rats and reduce the level of free radical and inflammatory factors.
At 6 h after transfusion and reperfusion, the biochemistry indices in serum of renal function in hemorrhagic shock rats were significantly increased than that of the sham group, thus promoting renal dysfunction in rats. However, the indices in the ligation group were significantly decreased than that of the shock group, although certain indices were still higher than that of the sham group. Moreover, histopathological observation also showed that the degree of necrosis in renal tubular epithelium and the amount of protein cast in tubular lumen were lower than that of the shock group. These results suggest that mesenteric lymph duct ligation can reduce renal dysfunction and injury in hemorrhagic shock rats.
Tissue reperfusion can lead to increased oxygen levels that can activate the complement system. This can lead to the stimulation of macrophage and polymorph nuclear (PMN) that further release cytokines and oxygen free radicals; the latter causing more serious cell injury, thus contributing to a vicious circle. This study showed that the shock group rats produced increased amounts of free radicals after shock and reperfusion as well as decreased levels of SOD. This called to attention that injury from free radicals played an important role in the pathogenesis of renal insufficiency induced by shock. Interestingly, in the ligation group, when the intestinal lymph fluid return was stopped by ligating the mesenteric lymph duct, the MDA content in renal homogenate reduced at various time points and the SOD activity enhanced. A possible mechanism could be that by ligating the mesenteric lymph duct, it cut off the BET induced by intestinal barrier dysfunction resulting from reperfusion, and through the mesenteric lymph blockade, the flow of toxic material to the whole body was stopped. Thus the cascade effect of toxin-cytokine and the effect of free radicals to tissue and cell injury were reduced. As a result, mesenteric lymph duct ligation attenuated the renal insufficiency induced by hemorrhagic shock efficiently.
The abnormal hemodynamics in the kidney, induced by microcirculatory disturbances from shock, is the major factor that causes kidney dysfunction. SIRS or compensatory anti-inflammatory response syndrome (CARS) is the result of massive release of inflammatory mediators, which are produced by PMN, and is one of the most important factors that contribute to the accelerated aggravation and organ function injury that contribute to the development of MODS.Citation9 TNFα and IL-6 are significant factors that lead to the uncontrolled release of proinflammatory cytokines.Citation10 MPO activity is an index of PMN activation. In this study, MPO activity and TNFα and IL-6 levels were significantly increased when the rats are in shock and at any point after transfusion and resuscitation. However, the MPO activity in the renal homogenate increased after blocking the mesenteric lymphatic fluid reflux by mesenteric lymph duct ligation, after both transfusion and resuscitationCitation11 with a gradual decrease in MPO activity as time progresses. TNFα and IL-6 levels were significantly lower than that of the shock group, which indicate that the intestinal lymphatic fluid reflux from hemorrhagic shock promotes the recruitment of PMN in kidney tissue thus promoting inflammation. While mesenteric lymph ligation prevented toxic material from intestine flowing to the whole body through the mesenteric lymph duct thus may reduce microcirculation obstacle, inhibit the recruitment of PMN, also stop the moving of TNFα and IL-6 induced by low-infusion in intestine and further reduce the cascade action of kidney tissue inflammatory cytokine,Citation12 efficiently against the kidney injury.
In conclusion, mesenteric lymph duct ligation can prevent the BET induced by intestine and stop toxic material from moving to the whole body through mesenteric lymph duct. This then decreases the cascade effect of toxin-mediated cytokines, improves blood circulation, reduces the recruitment of PMN, decreases the production and release of TNFα and IL-6, lowers the production of free radical and the consumption of SOD, thus preventing renal insufficiency induced by shock. These results indicate that mesenteric lymph fluid from shock plays an important role in shock-induced kidney dysfunction.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (30370561, 30770845, 30971203) and Natural Science Foundation of Hebei Province (C2004000649, C2008000503). No benefits in any form have been received or will be received from a commercial association related directly or indirectly to the subject of this article.
REFERENCES
- Mizock BA. The multiple organ dysfunction syndrome. Dis Mon. 2009;55(8):476–526.
- Barie PS, Hydo LJ, Pieracci FM, Shou J, Eachempati SR. Multiple organ dysfunction syndrome in critical surgical illness. Surg Infect (Larchmt). 2009;10(5):369–377.
- Xu DZ, Lu Q, Adams CA, Issekutz AC, Deitch EA. Trauma-hemorrhagic shock-induced up-regulation of endothelial cell adhesion molecules is blunted by mesenteric lymph duct ligation. Crit Care Med. 2004;32(3):760–765.
- Niu CY, Li JC, Zhao ZG, Zhang J, Shao XH. Effect of intestinal lymphatic circulation blockage in two-hit rats. World J Gastroenterol. 2006;12(36):5805–5812.
- Zhao ZG, Niu CY, Zhang J, Bo AH. Effects of mesenteric lymph duct ligation on apoptosis of renal tubule epithelial cells in rats after two-hits. Chin Crit Care Med. 2007;19(12):724–726.
- Nieuwenhuijzen GAP, Deitch EA, Gorsi RJA. The relationship between gut-derived bacteria and the development of the multiple organ dysfunction syndrome. J Anat. 1996;189(3):537–548.
- Gonzalez RJ, Moore EE, Biffl WL, Ciesla DJ, Silliman CC. The lipid fraction of post-hemorrhagic shock mesenteric lymph inhibits neutrophil apoptosis and enhances cytotoxic potential. Shock. 2000;14(3):404–408.
- Deitch EA, Adams CA, Lu Q, Xu DZ. Mesenteric lymph from rats subjected to trauma-hemorrhagic shock are injuric to rat pulmonary microvascular endothelial cells as well as human umbilical vein endothelial cell. Shock. 2001;16(4):290–293.
- Bone RC. Sirisaac newton, sepsis, SIRS and CARS. Crit Care Med. 1996;24(7):1125–1128.
- Wan L, Bellomo R, Di Giantomasso D, Ronco C. The pathogenesis of septic acute renal failure. Curr Opin Crit Care. 2003;9(6):496–502.
- Wang P, Li Y, Li J. Influence of hydroxyethyl starch on healing of colonic anastomosis in a rat model of peritonitis. J Invest Surg. 2009;22(5):375–382.
- Niu CY, Hou YL, Zhao ZG, Zhang YF, Ji JJ, Qiao HX, Zhang J, Yao YM. Role of intestinal lymphatic pathway in pathogenesis of intestine-derived bacteria/endotoxin translocation in rats in shock. Chin Crit Care Med. 2007; 19(5): 266–269.