Abstract
Hexachlorobutadiene (HCBD) is a potent nephrotoxin in rodents. Pharmacological studies have shown that pomegranate fruit preparations have antioxidant, anti-inflammatory chemopreventive effects. In this study, the effect of pomegranate seed oil (PSO) on HCBD-induced nephrotoxicity was investigated in adult male rats. Animals were divided into five groups. Group 1 was treated with corn oil (1 mL/kg, i.p.). Group 2 received a single dose of HCBD (50 mg/kg, i.p.). Groups 3–5 were treated with PSO (0.16, 0.32, and 0.64 mg/kg, i.p., respectively) 1 h before HCBD (50 mg/kg, i.p.) injection. A significant elevation of serum creatinine and urea (p < 0.001) levels as well as urine glucose and protein (p < 0.001) concentrations (as markers of acute renal failure) was observed 24 h after administration of HCBD as compared to control group. HCBD also caused a significant decrease in total thiol content (p < 0.001) and a significant increase in thiobarbituric acid reactive species (TBARS, as an index of lipid peroxidation) levels (p < 0.001) in kidney homogenate samples. PSO pretreatment resulted in a significant and dose-dependent decrease in serum creatinine (p < 0.001) and urea levels (p < 0.001) as well as urine glucose (p < 0.001) and protein concentrations (p < 0.001) when compared with HCBD treated alone. PSO also significantly reversed the HCBD-induced depletion in total thiol content (p < 0.001) and elevation in TBARS (p < 0.001) in kidney homogenate samples. The results of this study showed that PSO clearly attenuated HCBD-induced nephrotoxicity, but explanation and mechanism of this protection need further explorations.
INTRODUCTION
Hexachloro-1,3-butadiene (HCBD) is a halogenated aliphatic compound, mainly used as a solvent for elastomers and rubber compounds, heat-transfer liquid, transformer, and hydraulic fluids as well as an insecticide, herbicide, fungicide, and algicide. It is currently formed in considerable quantities as a by-product in the manufacture of chlorinated hydrocarbons such as tri- and tetrachloroethane and tetrachloromethane.Citation1,Citation2 HCBD is dispersed in the environment, and frequently human foods are contaminated with measurable quantities of HCBD. In addition, HCBD is found in drinking water. The solvent is also detected in aquatic organisms, birds, and mammals.Citation3 HCBD has deleterious health effects due to its toxicity and carcinogenicity.Citation4–7 HCBD is a potent nephrotoxin in rodents. Its bioactivation resulted in formation of toxic electrophilic metabolites which cause degeneration and necrosis in renal tubular epithelial cells.Citation8
Pomegranate, Punica granatum L., is an ancient medicinal food plant which natively grows from the Himalayas in northern India to Middle East but has also been cultivated and naturalized in many other regions including Mediterranean, Southeast Asia, tropical Africa, and American Southwest.Citation9 In addition to extensive uses of pomegranate in folk medicine of many cultures, pharmacological studies have shown that pomegranate fruit preparations have antioxidant and anti-inflammatory,Citation10,Citation11 antimicrobial,Citation12–15 anticancer, and chemopreventiveCitation16,Citation17 effects.
Plants have formed the basis of sophisticated traditional medicine systems which have given rise to some important drugs still in use today. The searches for new drug molecules, nowadays, guide the researchers across the globe toward natural products as an alternative source and class of medicinal compounds.Citation18 This study was designed to examine the influence of pomegranate seed oil (PSO) on HCBD-induced nephrotoxicity in rats.
MATERIALS AND METHODS
Adult male Wistar rats (Bu-Ali Research Institute, Mashhad, I. R. Iran), weighing 250–300 g, were used for all experiments. These animals were housed in a pathogen-free facility on a 12-hour light/dark schedule and with ad lib access to food and water. All animal procedures were approved by the university ethics committee and were in compliance with national laws and with National Institutes of Health guidelines for the use and care of laboratory animals.
DTNB (2,2′-dinitro-5,5′-dithiodibenzoic acid), TBA (2-thiobarbituric acid), n-butanol, NaOH (sodium hydroxide), NaCl (sodium chloride), Na2EDTA (ethylenediaminetetraacetic acid disodium salt), Trizma base (Tris (hydroxymethyl) aminomethane), phosphoric acid, HCl (hydrochloric acid), KCl (potassium chloride), ether, and TMP (tetramethoxypropane) were purchased from Merck (Darmstadt, Germany). HCBD was obtained from Fluka (St. Gallen, Switzerland). PSO (d = 0.81 g/mL at 25°C) was a gift from Urom Narin Co. (Uromeya, I. R. Iran).
After acclimatization, animals were randomly divided into five groups (six each) and individually put in the metabolic cages. Group 1 (control group) was treated with corn oil (1 mL/kg, i.p.). Group 2 was injected with a single dose of HCBD (50 mg/kg, i.p.). Groups 3–5 were treated with PSO (0.16, 0.32, and 0.64 mg/kg, i.p., respectively) 1 h before HCBD (50 mg/kg, i.p.) injection. All procedures were carried out between 10 and 12 am. The animals were killed 24 h after the injection of HCBD using ether anesthesia; blood samples were taken out by cardiac puncture for measuring the level of serum urea and creatinine. Twenty-four-hour urine samples were also collected and used for measuring glucose and protein concentration. In addition, the kidney tissues were homogenized in cold KCl solution (1.5%, pH 7) to give a 10% homogeny suspension and used for biochemical assays.
Urea concentration was determined colorimetrically by using Autoanalyzer (Technicon RA-1000, England) and urea kit (Man Lab Company, Tehran, I. R. Iran). Creatinine concentration was measured by the Jaffe's method.Citation19
Glucose concentration was estimated by the enzymatic assay (glucose oxidase) and protein concentration was measured by the turbidimetric method.Citation20,Citation21
The lipid peroxidation level of the kidney tissues was measured as malondialdehyde (MDA), which is the end product of lipid peroxidation and reacts with TBA as a thiobarbituric acid reactive substance (TBARS) to produce a red-colored complex which has peak absorbance at 532 nm.Citation22 Briefly, 3 mL phosphoric acid (1%) and 1 mL TBA (0.6%) were added to 0.5 mL of homogenate in a centrifuge tube and the mixture was heated for 45 min in a boiling water bath. After cooling, 4 mL of n-butanol was added to the mixture, vortexed for 1 min, and then centrifuged at 20,000 rpm for 20 min. The organic layer was transferred to a fresh tube and its absorbance was measured at 532 nm. The standard curve of MDA was constructed over the concentration range of 0–40 μM.Citation23
Total SH groups were measured using DTNB as the reagent. This reagent reacts with the SH groups to produce a yellow-colored complex which has peak absorbance at 412 nm. Briefly, 1 mL Tris–EDTA buffer (pH = 8.6) was added to 50 μL kidney homogenate in 2 mL cuvettes and sample absorbance was read at 412 nm against Tris–EDTA buffer alone (A1). Then 20 μL DTNB reagent (10 mM in methanol) was added to the mixture, and after 15 min (stored in laboratory temperature), the sample absorbance was read again (A2). The absorbance of DTNB reagent was also read as a blank (B). Total thiol concentration (mM) was calculated from the following equationCitation24:
Statistical analysis
Data were expressed as mean ± SEM. Statistical analysis was performed using one-way analysis of variance (ANOVA) followed by Tukey–Kramer post hoc test for multiple comparisons. The p-values less than 0.05 were considered to be statistically significant.
RESULTS
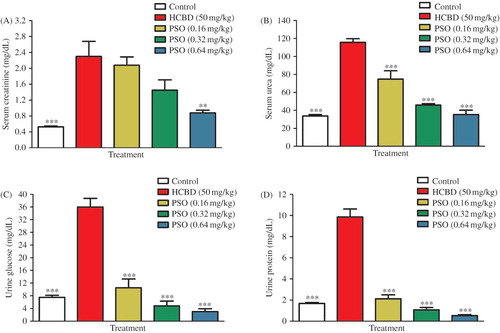
No observable toxicity or any gross changes in kidney tissues of animals pretreated with PSO were seen. A significant elevation of serum creatinine (3.4 fold, p < 0.001) and urea (2.4 fold, p < 0.001) levels as well as urine glucose (3.8 fold, p < 0.001) and protein (4.9 fold, p < 0.001) concentrations was observed 24 h after administration of HCBD as compared to those of control animals (–D). HCBD also caused a significant decrease in total thiol content (50.8%, p < 0.001) and a significant increase in TBARS levels (1.3 fold, p < 0.001) in kidney homogenate samples (, F). PSO pretreatment resulted in a significant and dose-dependent decrease in serum creatinine (0.88 ± 0.07 vs. 2.30 ± 0.38 mg/dL, p < 0.001; 0.64 mg/kg) and urea (35.33 ± 4.89 vs. 115.75 ± 3.97 mg/dL, p < 0.001; 0.64 mg/kg) levels as well as urine glucose (3.00 ± 0.93 vs. 36.00 ± 2.74 mg/dL, p < 0.001; 0.64 mg/kg) and protein concentrations (0.52 ± 0.10 vs. 9.85 ± 0.76 mg/dL, p < 0.001; 0.64 mg/kg), when compared with HCBD alone (–D). PSO also significantly reversed the HCBD-induced depletion in total thiol content (0.53 ± 0.02 vs. 0.29 ± 0.02 mM, p < 0.001; 0.64 mg/kg) and elevation in TBARS levels (33.83 ± 2.65 vs. 81.17 ± 3.57 nmol/g tissue, p < 0.001; 0.64 mg/kg) in kidney homogenate samples (, F).
FIGURE 1. Effect of pomegranate seed oil (PSO) on concentration of serum creatinine (A), serum urea (B), urinary glucose (C), urinary protein (D), renal malondialdehyde (E), and total thiol (F) contents in male rats treated with hexachlorobutadiene. All injections were carried out intraperitoneally. PSO was injected 1 h before HCBD. Data shown as mean ± SEM (n = 6).
Preliminary experiment showed that PSO alone did not significantly modify these biochemical parameters.
DISCUSSION
It is believed that natural compounds and their derivatives represent a source of potential chemotherapeutic agents. Dietary supplementation with these products rich in antioxidants is associated with inhibition of toxicity of many chemicals.Citation18 The results obtained in this study suggested that PSO has an overall protective effect against HCBD-induced nephrotoxicity in rat model. The observed protective effects can be attributed to the antioxidant properties of PSO.
This study showed that HCBD with a dose of 50 mg/kg could induce renal dysfunction as revealed by increased urinary excretion of glucose and protein and elevated levels of serum urea and creatinine. These data agreed with our previous findings.Citation25,Citation26 Age, sex, and strain are well-known factors conditioning nephrotoxicity caused by HCBD in rats.Citation27 Therefore, only young male adult rats (10 weeks) were used. Pretreatment with PSO completely restored the altered renal function tests in a dose-dependent manner. The antioxidant status of kidney is also significantly lowered in the HCBD-alone-treated rats. This finding is inconsistent with previous reports.Citation28,Citation29 Actually, the increase in TBARS levels and decrease in total thiol contents suggest enhanced oxidative stress causing tissue damage and renal functional failure. On the other hand, PSO pretreatment resulted in a significant and dose-dependent improvement in the renal antioxidant status.
Sulfhydryl (SH) groups are highly reactive constituents of protein and non-protein molecules (e.g., glutathione, GSH, or thioredoxin), and they participate in important biochemical and metabolic processes such as redox signaling, detoxification mechanisms, maintenance of protein systems, and function of metabolic enzymes. They are also important scavengers of oxygen-derived free radicals.Citation30,Citation31 In this study, the thiol content, which is well known to be depleted following the toxicity of many haloalkenes,Citation32,Citation33 was measured as an indicator of HCBD-induced nephrotoxicity. HCBD caused significant SH depletion in the kidney homogenate samples while PSO pretreatment resulted in a significant improvement. Several authors investigated the thiol contents (especially non-protein) in the liver and kidney of HCBD-treated rats. Lock and Ishmael reported that single i.p. administration of HCBD to male rats at 300 mg/kg produced a marked decrease in the total non-protein SH content (NP-SH) of the liver, whereas renal NP-SH remained unchanged.Citation34 On the contrary, Kluwe et al. showed that single i.p. injection of HCBD decreased hepatic and renal NP-SH concentrations in mice in a dose-related manner.Citation35 According to the Hook et al., treatment of rats with diethyl maleate, a compound which decreases tissue NS-PH content, markedly potentiated the nephrotoxicity of HCBD.Citation27 The inhibitory effect of HCBD on renal glutathione reductase in rat has also been reported.Citation36 Recently, Trevisan et al. found that, unlike liver, kidney GSH content significantly increased 24 and 48 h after treatment with HCBD in male rats but not in female rats or in renal cortical slices, in vitro.Citation3
PSO is a rich source of conjugated fatty acids of which punicic acid is the most common.Citation37,Citation38 Polyphenolic compounds are also present in the seed oil of the pomegranate.Citation10,Citation38 These components from PSO have antioxidant and anti-inflammatory activities by inhibiting pro-inflammatory enzymes and expression of pro-inflammatory cytokines.Citation10,Citation39,Citation40 PSO has also been shown in experimental studies to enhance B-cell function in vivo,Citation41 to suppress proliferation of several different tumor cell types in vitro,Citation16,Citation42–44 and to reduce skin carcinogenesis in mice,Citation45 mammary carcinogenesis in a mouse mammary organ culture model, and colon carcinogenesis in rats.Citation46 Pomegranate seed also contains coniferyl 9-O-[beta-d-apiofuranosyl(1–6)]-O-beta-d-glucopyranoside, sinapyl 9-O-[beta-d-apiofuranosyl (1-6)]-O-beta-d-glucopyranoside, sterols (daucosterol, beta-sitosterol), and hydroxybenzoic acids (gallic, ellagic and its derivatives) which exhibited antioxidant activity and decreased lipid peroxidation.Citation11,Citation47 Vivancos and Moreno showed that beta-sitosterol reverts impaired glutathione/oxidized glutathione ratio and modulates antioxidant enzyme response in RAW 264.7 macrophage.Citation48
Several hypotheses may explain the protective effect of PSO against HCBD-induced nephrotoxicity. As mentioned before, the renal toxicity of HCBD is due to bioactivation by glutathione-S-conjugate formation. Subsequently, further processing by the enzymes of the mercapturic acid pathway leads to formation of toxic electrophilic intermediates which damage renal epithelial cells by a combination of covalent modification of macromolecules, thiol depletion, and initiation of lipid peroxidation.Citation8,Citation25,Citation29,Citation40,Citation44,Citation49 PSO may decrease HCBD-induced nephrotoxicity by quenching these toxic metabolites. Other possibilities are the inhibitory effects of PSO on enzymes involved in the bioactivation of HCBD such as glutathione-S-transferase (GST) or cysteine-S-conjugate β-lyase. Recently, decreased GST activity and transcription were observed in mice that ingested pomegranate. The authors claimed that GST inhibition could reflect the decrease in protein damage, which will, most likely, translate into less GST activity.Citation50 The inhibitory effects of plant polyphenol on GST(s) has also been shown in vitro.Citation51 Experimental studies have also demonstrated that inhibitors of cysteine-S-conjugate β-lyase (such as aminooxyacetic acid) reduced HCBD-induced nephrotoxicity.Citation52,Citation53
CONCLUSION
In conclusion, the results of this study showed that PSO clearly attenuated HCBD-induced nephrotoxicity. This is supported by improvements of renal functional tests and by the decrease of proteins and lipids damage, but explanation and mechanism of this protection need further explorations.
Acknowledgment
This investigation was financially supported by the Vice-Chancellor for Research, Mashhad University of Medical Sciences, Mashhad, Iran.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Anonymous. Chemical review, hexachloro-1, 3-butadiene. Dangerous Properties Ind Mater Rep. 1992;12:2–23.
- Verschueren K. Handbook of Environmental Data on Organic Chemicals. 3rd ed. New York: Van Nostrand Reinhold; 1996:1070–1072.
- Trevisan A, Cristofori P, Beggio M, Venturini MB, Di Marco L, Zanetti E. Segmentary effects on the renal proximal tubule due to hexachloro-1,3-butadiene in rats: biomarkers related to gender. J Appl Toxicol. 2005;25:13–19.
- Kociba RJ, Keyes DG, Jersey GC, Results of a two year chronic toxicity study with hexachloro-1, 3-butadiene in rats. Am Ind Hyg Assoc J. 1977;38:589–602.
- Kociba RJ, Schwetz BA, Keyes DG, Chronic toxicity and reproduction studies of hexachloro-1, 3-butadiene in rats. Environ Health Perspect. 1977;21:49–53.
- Choudhary G. Human health perspectives on environmental exposure to HCBD: A review. Environ Carcinogen Ecotoxicol Rev. 1995;C13(2):179–203.
- Green T, Lee R, Farrar D, Hill J. Assessing the health risks following environmental exposure to hexachloro–1, 3–butadiene. Toxicol Lett. 2003;138:63–73.
- Lock EA, Ishmael J. The acute toxic effects of hexachloro–1: 3–butadiene on the rat kidney. Arch Toxicol. 1979;43:47–57.
- Jurenka J. Therapeutic applications of pomegranate (Punica granatum L.): A review. Altern Med Rev. 2008;13(2):128–144.
- Schubert SY, Lansky EP, Neeman I. Antioxidant and eicosanoid enzyme inhibition properties of pomegranate seed oil and fermented juice flavonoids. J Ethnopharmacol. 1999;66:11–17.
- Lansky P, Newman RA. Punica granatum (pomegranate) and its potential for prevention and treatment of inflammation and cancer. J Ethnopharmacol. 2007;109:177–206.
- Braga LC, Shupp JW, Cummings C, Pomegranate extract inhibits Staphylococcus aureus growth and subsequent enterotoxin production. J Ethnopharmacol. 2005;96:335–339.
- Menezes SM, Cordeiro LN, Viana GS. Punica granatum (pomegranate) extract is active against dental plaque. J Herb Pharmacother. 2006;6(2):79–92.
- Khalil EA. Antidiabetic effect of an aqueous extract of pomegranate (Punica granatum L) peels in normal and alloxan diabetic rats. Egyptian J Hosp Med. 2004;16:92–99.
- Huang TH, Peng G, Kota BP, Antidiabetic action of Punica granatum flower extract: activation of PPAR-gamma and identification of an active component. Toxicol Appl Pharmacol. 2005;207:160–169.
- Albrecht M, Jiang W, Kumi-Diaka J, Pomegranate extracts potently suppresses proliferation, xenograft growth, and invasion of human prostate cancer cells. J Med Food. 2004;7:274–283.
- Adams L, Seeram NP, Aggarwal BB, Takada Y, Sand D, Heber D. Pomegranate juice, total pomegranate tannins and punicalagin suppress inflammatory cell signaling in colon cancer cells. J Agric Food Chem. 2006;54:980–985.
- Gurib-Fakim A. Medicinal plants: Traditions of yesterday and drugs of tomorrow. Mol. Aspects Med. 2006;27:1–93.
- Masson P, Ohlsson P, Bjorkhem I. Combined enzymatic-Jaffe's method for determination of creatinine in serum. Clin Chem. 1981;27:18–21.
- Lott JA, Turner K. Evaluation of Trinder's glucose oxidase method for measuring glucose in serum and urine. Clin Chem. 1975;21:1754–1760.
- Mc Elderry LA, Tarbit IF, Cassells-Smith AJ. Six methods for urinary protein compared. Clin Chem. 1982;28:356–360.
- Fernandez J, Perez-Alvarez JA, Fernandez-lopez JA. Thiobarbituric acid test for monitoring lipid oxidation in meat. Food Chem. 1997;99:345–353.
- Hosseinzadeh H, Sadeghnia HR, Ziaee T, Danaee A. Protective effect of aqueous saffron extract (Crocus sativus L.) and crocin, its active constituent, on renal ischemia-reperfusion-induced oxidative damage in rats. J Pharm Pharm Sci. 2005;8:387–393.
- Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal Biochem. 1968;25:192–205.
- Boroushaki MT, Mofidpour H, Sadeghnia HR. Protective effect of safranal against hexachloro-1, 3-butadiene-induced nephrotoxicity in rat. Iran J Med Sci. 2007;32:173–176.
- Boroushaki MT, Mofid Pour H, Dolati K. Protective effects of verapamil against hexachlorobutadiene nephrotoxicity in rat. Iran J Med Sci. 2004;29:101–104.
- Hook JB, Ishmael J, Lock EA. Nephrotoxicity of hexachloro-1:3-butadiene in the rat: The effect of age, sex, and strain. Toxicol Appl Pharmacol. 1983;67(1):122–131.
- Dekant W, Vamvakas S, Anders MW. Bioactivation of hexachloro-1, 3-butadiene by glutathione conjugation. Food Chem Toxicol. 1990;28:285–293.
- Kim HS, Cha SH, Abraham DG, Cooper AJ, Endou H. Intranephron distribution of cysteine S-conjugate β-lyase activity and its implication for hexachloro-1,3-butadiene-induced nephrotoxicity in rats. Arch Toxicol. 1997;71:131–141.
- Ziegler DM. Role of reversible oxidation-reduction of enzyme thiols-disulfides in metabolic regulation. Ann Rev Biochem. 1985;54:305–329.
- Meyer AJ, Hell R. Glutathione homeostasis and redox-regulation by sulfhydryl groups. Photosynth Res. 2005;86:435–457.
- Chen Q, Jones TW, Brown PC, Stevens JL. The mechanism of cysteine conjugate cytotoxicity in renal epithelial cells. Covalent binding leads to thiol depletion and lipid peroxidation. J Biol Chem. 1990;265:21603–21611.
- Cooper AJ, Bruschi SA, Anders MW. Toxic, halogenated cysteine S-conjugates and targeting of mitochondrial enzymes of energy metabolism. Biochem Pharmacol. 2002;15:64(4):553–564.
- Lock EA, Ishmael J. Hepatic and renal nonprotein sulfhydryl concentration following toxic doses of hexachloro-1,3-butadiene in the rat: The effect of Aroclor 1254, phenobarbitone, or SKF 525A treatment. Toxicol Appl Pharmacol. 1981;57(1):79–87.
- Kluwe WM, McNish R, Smithson K, Hook JB. Depletion by 1,2-dibromoethane, 1,2-dibromo-3-chloropropane, tris (2,3-dibromopropyl) phosphate, and hexachloro-1,3-butadiene of reduced non-protein sulfhydryl groups in target and non-target organs. Biochem Pharmacol. 1981;30(16):2265–2271.
- Lock EA, Schnellmann RG. The effect of haloalkene cysteine conjugates on rat renal glutathione reductase and lipoyl dehydrogenase activities. Toxicol Appl Pharmacol. 1990;104(1):180–190.
- Fadavi A, Barzegar M, Azizi MH. Determination of fatty acids and total lipid content in oil seed of 25 pomegranates varieties grown in Iran. J Food Comp Anal. 2006;19:676–680.
- Kaufman M, Wiesman Z. Pomegranate oil analysis with emphasis on MALDI-TOF/MS triacylglycerol fingerprinting. J Agric Food Chem. 2007;55:10405–10413.
- Singh RP, Murthy KNC, Jayaprakasha GK. Studies on the antioxidant activity of pomegranate (Punica granatum) peel and seed extracts using in vitro models. J Agric Food Chem. 2002;50:81–86.
- Rasheed Z, Akhtar N, Anbazhagan AN, Ramamurthy S, Shukla M, Haqqi TM. Polyphenol-rich pomegranate fruit extract (POMx) suppresses PMACI-induced expression of pro-inflammatory cytokines by inhibiting the activation of MAP kinases and NF-kappaB in human KU812 cells. J Inflamm. 2009;6(1):1–30.
- Yamasaki M, Kitagawa T, Koyanagi N, Dietary effect of pomegranate seed oil on immune function and lipid metabolism in mice. Nutrition. 2006;22:54–59.
- Kim ND, Mehta R, Yu W, Chemopreventive and adjuvant therapeutic potential of pomegranate (Punica granatum) for human breast cancer. Breast Cancer Res Treat. 2002;71: 203–217.
- Kawaii S, Lansky EP. Differentiation-promoting activity of pomegranate (Punica granatum) fruit extracts in HL-60 human promyelocytic leukemia cells. J Med Food. 2004;7:13–18.
- Khan N, Hadi N, Afaq F, Syed DN, Kweon MH, Mukhtar H. Pomegranate fruit extract inhibits prosurvival pathways in human A549 lung carcinoma cells and tumor growth in athymic nude mice. Carcinogenesis. 2007;28:163–173.
- Hora JJ, Maydew ER, Lansky EP, Dwivedi C. Chemopreventive effects of pomegranate seed oil on skin tumor development in CD1 mice. J Med Food. 2003;6:157–161.
- Kohno H, Suzuk R, Yasui Y, Hosokawa M, Miyashita K, Tanaka T. Pomegranate seed oil rich in conjugated linolenic acid suppresses chemically induced colon carcinogenesis in rats. Cancer Sci. 2004;95:481–486.
- Wang RF, Xie WD, Zhang Z, Bioactive compounds from the seeds of Punica granatum (pomegranate). J Nat Prod. 2004;67(12):2096–2098.
- Vivancos M, Moreno JJ. Beta-Sitosterol modulates antioxidant enzyme response in RAW 264.7 macrophages. Free Radic Biol Med. 2005;39:91–97.
- Reichert D, Schutz S. Mercapturic acid formation is an activation and intermediary step in the metabolism of hexachloro-1, 3-butadiene. Biochem Pharmacol. 1986;35:1271–1275.
- Faria A, Monteiro R, Mateus N, Azevedo I, Calhau C. Effect of pomegranate (Punica granatum) juice intake on hepatic oxidative stress. Eur J Nutr. 2007;46:271–278.
- Das M, Bickers DR, Mukhtar H. Plant phenols as in vitro inhibitors of glutathione S-transferase(s). Biochem Biophys Res Commun. 1984;12:427–433.
- Pratt IS, Lock EA. Deacetylation and further metabolism of the mercapturic acid of hexachloro-1,3-butadiene by rat kidney cytosol in vitro. Arch Toxicol. 1988;62(5):341–345.
- de Ceaurriz J, Ban M. Role of gamma-glutamyltranspeptidase and beta-lyase in the nephrotoxicity of hexachloro-1,3-butadiene and methyl mercury in mice. Toxicol Lett. 1990; 50(2–3):249–256.
