Abstract
Background/aims: Silymarin is an herbal extract with antioxidant properties that can reduce oxidative stress-mediated injuries in murine models of liver, heart, and kidney diseases. Silymarin can also increase p53-mediated cellular apoptosis in vitro. We tested the effect of silymarin administration before glycerol-induced acute kidney injury (Gly-AKI) in rats. Methods: Renal function, tubular injury, oxidative stress, leukocytes infiltration, and renal expression of apoptosis regulating proteins (p53, p-p53, Bax, Bcl-2, survivin, and cleaved caspase-3) were evaluated 6 or 24 h after glycerol. Results: Silymarin exacerbated the renal impairment and tubular apoptosis but had no effect on tubular necrosis or renal leukocytes infiltration. Renal lipid and DNA peroxidation was increased after glycerol and silymarin did not reduce oxidative stress. Proteins p53, p-p53, and proapoptotic Bax were upregulated in Gly-AKI rats treated with silymarin, whereas anti-apoptotic Bcl-2 was reduced in this group. Cleaved caspase-3 was overexpressed in Gly-AKI rats, particularly when treated with silymarin. Survivin was less expressed in Gly-AKI than in controls, but this deficit was not aggravated by silymarin. Conclusion: The persistence of oxidative stress, inflammatory reaction, and tubular necrosis, as well as exacerbation of p53-mediated tubular apoptosis, led to a more severe renal impairment in Gly-AKI rats treated with silymarin.
INTRODUCTION
Silymarin is an herbal preparation extract from milk thistle comprising 60–85% of flavanolignans (mainly silibinin), fatty acids, polyphenolic compounds, and small amounts of flavonoids.Citation1 Silymarin administration to rats during oxidative stress conditions restores the endogenous antioxidant enzymes (superoxide dismutase, catalase, glutathione peroxidase), maintains reduced glutathione (GSH) and nonenzymatic antioxidant levels (tocopherol, carotene, and ascorbate), enhancing tissue resistance to oxidative stress.Citation2–4 Silymarin and silibinin have also been evaluated as anti-cancer agents. Several studies have shown their capacity to decrease tumor cell proliferation and induce apoptosis in cancer cell lineages, endothelial cells, or normal cells exposed to carcinogenic stimuli.Citation5–9 The cornerstone of the proapoptotic action of silymarin/silibinin is the activation of the tumor suppressor gene p53.Citation5–9 Several downstream effectors of p53 activation have been implicated in silymarin-induced apoptosis, such as increased expression of proapoptotic Bax protein as well as reduction of the anti-apoptotic proteins Bcl-2, Bcl-xl, and survivin.Citation6–9
In this study, we evaluated the effects of silymarin treatment on renal function and histopathology in glycerol-induced acute kidney injury (Gly-AKI) in rats. Renal function was evaluated 24 h after glycerol injection through the estimation of the glomerular filtration rate (creatinine clearance). This AKI model (Gly-AKI) is characterized by renal ischemia and myoglobin-derived heme-iron-mediated renal oxidative stressCitation10–14 leading to severe tubular injury (necrosis and apoptosis), renal and systemic inflammatory response, preglomerular vasoconstriction, and acute renal failure.Citation15–17 Based on the previous studies in rats subjected to renal ischemia/reperfusion injury,Citation18,Citation19 we expected that silymarin would have antioxidant and protective effect in Gly-AKI rats. The initial purpose of this experimental study was to evaluate the potential of this herbal extract in the renal protection after rhabdomyolysis. Surprisingly, silymarin aggravated the renal impairment in this study suggesting the preponderance of its proapoptotic effect in our model. To confirm this impression, we carefully analyzed the tubular injury, oxidative stress, inflammatory reaction, and p53-mediated tubular apoptosis in response to silymarin treatment in Gly-AKI rats.
METHODS
Induction of Gly-AKI and animal treatment
Male Wistar rats (200–250 g) were bred in the animal facility at our institution. The experiments were performed in accordance with the guidelines established by the Brazilian College for Animal Experimentation. Gly-AKI was induced after overnight fast by a single intramuscular injection of 50% glycerol (7 mL/kg) divided into both lower hind limbs. Control rats received saline injection in place of glycerol (sham). After glycerol injection, rats had free access to rat chow and tap water. Silymarin (Sigma, St. Louis, Michigan, USA) diluted in 1% dimethyl sulfoxide (DMSO)Citation20 was injected intraperitoneally (100 mg/kg/dose) concomitant with glycerol injection and 3 h after.Citation21,Citation22 The groups were control (sham + vehicle), control + silymarin, Gly-AKI (glycerol + vehicle), and Gly-AKI + silymarin (n = 7–13 rats/group). Serum creatine phosphokinase (CK) concentration 6 h after glycerol injection was evaluated in rats from all groups to assure that silymarin treatment had no influence on the rhabdomyolysis induced by the glycerol injection.
Creatinine clearance
For this procedure, 22 h after glycerol injection rats were expanded with water (10% body weight) by gavage followed by collection of 2 h spontaneously voided urine. At the end of urine collection, blood was drawn through cardiac puncture. Serum creatinine and urinary creatinine were measured by automated analyzer (Cobas–Mir, Roche, Basel, Switzerland). Creatinine clearance was calculated and normalized to 100 g body weight.
Histological analysis
Paraffin-embedded slides from kidneys harvested 24 h after glycerol injection were stained with hematoxylin and eosin. The number of totally necrotic and desquamated tubules (absence of nucleus) was quantified in 15 random cortical high power fields (HPF 400X) for each rat.
Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling assay
In situ detection of DNA fragmentation characteristic of apoptotic cells was performed using terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) method. Briefly, longitudinal sections of the paraffin-embedded kidneys were dewaxed, and endogenous peroxidase was blocked in 3% hydrogen peroxide. The tissue was incubated with Proteinase K (Roche, Mannheim, Germany) 20 mg/mL for 15 min at room temperature, then rinsed in terminal deoxynucleotidyl transferase buffer (30 mM Tris, 140 mM sodium cacodylate, 1 mM cobalt chloride, pH 7.2), incubated with terminal deoxynucleotidyl transferase (Amersham Biosciences, Piscataway, New Jersey, USA) 1:50, and biotinylated deuridine triphosphate (Gibco, Grand Island, New York, USA) 1:50 in terminal deoxynucleotidyl transferase buffer for 60 min at room temperature. Labeled nuclei were detected with Vectastain ABC (Vector Laboratories, Burlingame, California, USA) incubation for 30 min developed by diaminobenzidine tetrahydrochloride (Dako, Carpinteria, California, USA) as a substrate chromogen solution. Sections were counterstained with hematoxylin. Positive tubular cells were counted in 10 random HPF (X400) in the cortex and 10 in the medulla.
Immunohistochemistry: Macrophages (anti-ED-1), T lymphocytes (anti-CD43), DNA oxidation (8-OHdG), cleaved caspase-3, and survivin
Kidneys were harvested at appropriate times, fixed in 10% formalin (except 8-OHdG, fixed in methacarn), and embedded in paraffin. Slides were dewaxed, endogenous peroxidase blocked in 3% H2O2, antigen retrieved heating tissue at 93˚C in a microwave oven in 10 mM citrate buffer, pH 6 for 10 min (CD43 and 8-OHdG), 20 min (cleaved caspase-3 and survivin), or 30 min (ED-1). After unspecific blocking, the slides were incubated overnight at 4˚C with primary antibodies (anti-ED-1 1:100, Serotec, Oxford, UK; anti-CD43 1:50, Accurate Chemical, Wesbury, New York, USA; anti-8-OHdG 1:100, Japan Institute for the Control Aging, Japan; anti-cleaved caspase-3 1:400, Cell Signaling Technology, Massachusetts, USA; anti-survivin 1:50, Santa Cruz Biotechnology, California, USA). In the next morning, the procedure was completed by incubations with specific biotinylated secondary antibodies (1:200 survivin, CD4, cleaved caspase-3, and 8-OHdG, 1:400 ED-1), ABC detection kit (Dako, Glostrup, Denmark), and diaminobenzidine tetrahydrochloride color developer (Dako, Carpinteria, California, USA). Leukocytes and positive-cleaved caspase-3 cells were counted in 10 random cortical and 10 medullar tubulointerstitial HPF (X400). Positive 8-OHdG tubular cell nuclei were counted in 10 cortical HPF (X400). To evaluate survivin expression, 10 deep cortex HPF X400/rat were grid lined 10 × 10 and the percentage of positive grid field/HPF (%) was counted.
Lipid peroxidation
Lipid peroxidation was determined by malondialdehyde (MDA) concentration in renal tissue 6 h after glycerol injection. Samples of 250 mg of renal cortex were homogenated in 5 mL of ice-cold 1.15% KCl, and 0.5 mL aliquot of the homogenate was mixed with 3 mL of 1% phosphoric acid and 1 mL of 0.6% thiobarbituric acid. The mixture was heated for 45 min in a boiling water bath and after addition of 4 mL of n-butanol it was centrifuged at 2000 g for 20 min. The absorbance of the upper organic layer was read at 535 nm by a spectrophotometer (Beckman DU 530, California, USA) and compared with a standard curve of freshly prepared tetraethoxypropane at concentrations of 5.12, 10.25, and 20.5 nmol/mL.Citation23 Values of the concentration of MDA were corrected for kidney weight.
Western immunoblotting for p53, p-p53, Bax, Bcl-2
Renal fragments from outer medulla were collected 6 h after glycerol injection and homogenized at 4˚C in 30 mM Tris HCl pH 7.5, 10 mM ethylene glycol tetracetic acid (EGTA), 5 mM ethylenediamine tetracetic acid (EDTA), 1 mM dithiothreitol (DTT), 250 mM sucrose, and 40 μL/mL cocktail of protease inhibitors (Complete Mini, Roche, Mannheim, Germany). The homogenized solution was centrifuged at 11,000 g at 4˚C for 10 min. A sample of the supernatant was used for protein quantification using Bradford method (Bio Rad protein assay, Hercules, California, USA). Fifty micrograms of total protein in 5% glycerol, 0.03% bromophenol blue, and 10 mM DTT were loaded in 10% (p53, p-p53) or 12.5% (Bax, Bcl-2) sodium dodecyl sulfate (SDS)-polyacrylamide gel and electrophoresed in Laemmli solution. Molecular weight marker (Rainbow, Amersham, Pharmacia, New Jersey, USA) was used as standard. Proteins were transferred to nitrocellulose membranes (Bio-Rad, Hercules, California, USA). Nonspecific binding was blocked by incubating the membranes overnight 4˚C (p53) or for 1 h (p-p53, Bax.Bcl-2) with 5% nonfat milk at room temperature. Primary antibodies incubated for 1 h at room temperature (goat anti-rat p53, Santa Cruz, California, USA) or overnight at 4˚C [rabbit anti-rat p-p53 (Ser 15), Cell Signaling Technology, Massachusetts, USA; rabbit anti-rat Bax, Cell Signaling Technology, Massachusetts, USA; and mouse anti-rat Bcl-2, Santa Cruz Biotechnology, California, USA]. After washing with buffer, horseradish peroxidase (HRP)-conjugated secondary antibodies 1:40000 (p53), 1:2000 (p-p53), 1:250 (Bax), and 1:5000 (Bcl-2) were incubated for 1 h at room temperature. Immunoreactive bands were visualized using the enhanced chemiluminescence method (Super Signal CL-HRP Substrate System, Illinois, USA). Immunoblot for β-actin (Santa Cruz Biotechnology, California, USA) was used as load control. Quantification of the bands was done by optical densitometry using Image J software, version 1.37 (National Institute of Health, Bethesda, Maryland, USA).
Statistical analysis
Data are expressed as mean ± SEM. Analysis of variance and Student–Newman–Keuls tests were used to test parametric values and Mann–Witney or Kruskal–Wallis–Dunn for nonparametric values. Data association was tested using linear regression. p-Value less than 0.05 was considered significant.
RESULTS
Effects of silymarin on renal function and tubular injury in Gly-AKI rats
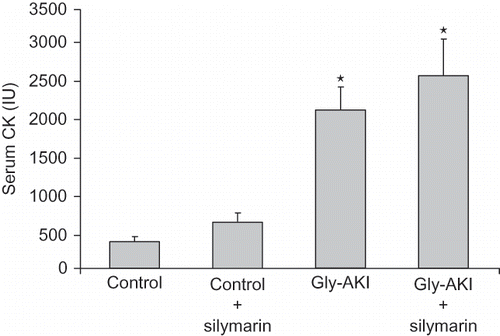
We observed a significant increase in the serum creatinine in the Gly-AKI rats 24 h after glycerol injection. The serum creatinine elevation was exacerbated by silymarin treatment. The evaluation of glomerular filtration rate by creatinine clearance 24 h after glycerol confirmed that silymarin treatment exacerbated the renal impairment in Gly-AKI rats (). There was no difference in the intensity of rhabdomyolysis between Gly-AKI rats, as determined by serum CK levels 6 h after glycerol injection ().
TABLE 1. Renal function 24 h after glycerol injection
FIGURE 1. Serum creatinine phosphokinase (CK) level 6 h after glycerol injection. There was a significant increase in serum CK after glycerol injection. Silymarin administration had no significant influence on CK rise, which excludes any interference in the glycerol-induced rhabdomyolysis. n = 6 rats/group.
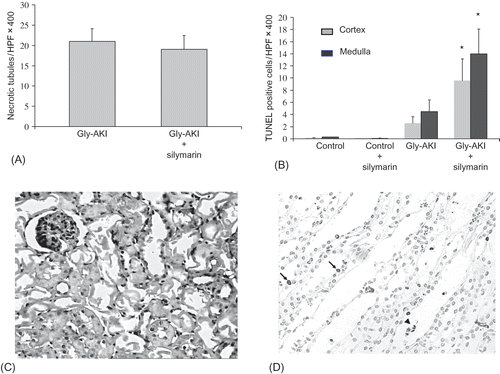
The histological analysis (hematoxylin-eosin) 24 h after glycerol, using an objective quantification of the amount of totally denuded cortical tubules/HPF, showed intense but similar prevalence of necrotic tubules in treated and untreated Gly-AKI rats (A and C). The TUNEL staining at 24 h showed increased number of apoptotic tubular cells, predominating in the outer medulla in Gly-AKI. Silymarin significantly increased the number of TUNEL positive cells both in the renal cortex and in the outer medulla (B and D).
FIGURE 2. Histological analysis of the renal tissue 24 h after glycerol injection. (A) Quantification of the number of cortical necrotic tubules/HPF showing 21.1 ± 2.8 (31% of the total number of tubules/HPF X400) and 19.2 ± 2.4 (28% of total number of tubules/HPF X400), respectively, in Gly-AKI and Gly-AKI + sily. Difference not significant: n = 8 (Gly-AKI) and 12 (Gly-AKI + sily). (B) TUNEL staining for the detection of apoptotic cells showing increased tubular apoptosis mainly in the outer medulla in Gly-ARF rats that was significantly accentuated by silymarin treatment. (C) Representative HE stained slide of the renal cortex from Gly-AKI rat, showing several totally denuded necrotic tubules. (D) Outer medulla field of a representative Gly-AKI + silymarin rat showing several apoptotic tubular cells lining the basement membrane (arrow) or desquamated into the tubular lumen (head arrow). n = 5–9/rats/group.
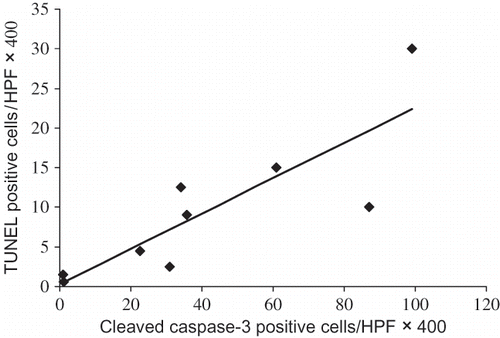
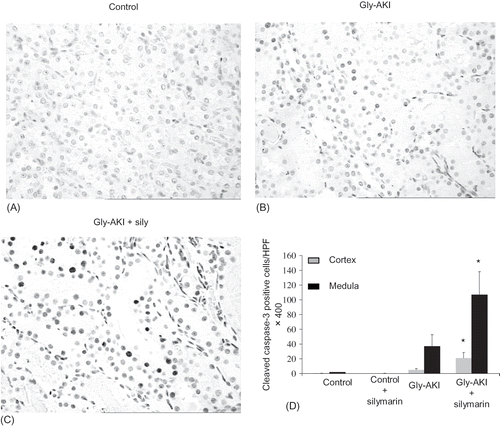
The renal expression of cleaved caspase-3 was evaluated by immunohistochemistry 24 h after glycerol injection. We observed a nuclear pattern of positive signals in tubular cells as had already been described by others.Citation24–26 The expression was increased in Gly-AKI (B) and significantly exacerbated by silymarin treatment (C). The quantitative analysis confirmed statistically significant increase in the expression of cleaved caspase-3 in Gly-AKI + silymarin (D). We also observed a significant positive correlation between positive cleaved caspase-3 cells and TUNEL positive cells in Gly-AKI rats subjected to both staining in separate experiments ().
FIGURE 3. Cleaved caspase-3 expression (immunohistochemistry) 24 h after glycerol injection. Positive cells showed nuclear staining and were more prevalent in Gly-AKI rats as compared with controls, predominated in outer medulla in relation to cortex and the expression was intensely exacerbated by silymarin treatment (darker nuclei). (A) Representative slide of the outer medulla of a health control rat, (B) Gly-AKI rat, (C) Gly-AKI rat treated with silymarin, and (D)quantification of positive caspase-3 cells/ HPF X400. n =5–6 rats/group.
Renal tissue peroxidation
The lipid peroxidation was evaluated by MDA level in the renal cortex 6 h after glycerol injection. There was a moderate but significant increase in the renal MDA concentration in Gly-AKI rats. The silymarin treatment was not able to block the MDA increase after glycerol injection ( A).
FIGURE 5. Renal tissue peroxidation after glycerol injection. (A) Gly-AKI rats showed increased level of cortical MDA 6 h after glycerol denoting increased lipid peroxidation. Silymarin treatment could not mitigate the lipid oxidative stress. n = 5–6 rats/group. (B) Similarly the DNA oxidative damage evaluated by 8-OHdG expression in the renal cortex 24 h after glycerol was increased in Gly-AKI rats and was not attenuated by silymarin treatment. (C) Representative slide of 8-OHdG immunohistochemistry in control and (D) Gly-AKI rat (X400). Note positive nuclei (darker) particularly in the more damaged cortical tubules. n = 6–7 rats/group.
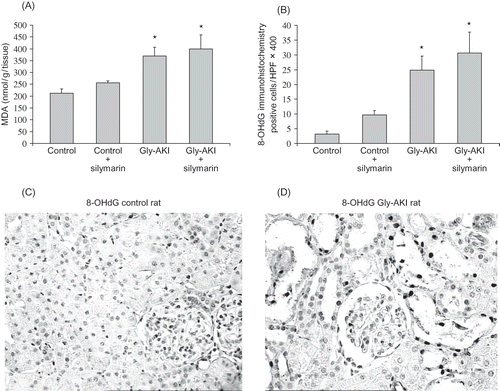
DNA oxidation was evaluated by renal immunohistochemistry 24 h after glycerol injection. This assay identifies the nuclear expression of 8-OHdG, a metabolite derived from base reaction with free radicals. Oxidative DNA damage was increased in the renal cortex of Gly-AKI rats as compared with controls. The silymarin treatment could not attenuate the DNA damage (B). C and D are examples of, respectively, control and Gly-AKI 8-OHdG immunohistochemistry.
Leukocytes infiltration in the renal tissue
The leukocytes infiltration in the renal tissue was evaluated by immunohistochemistry using antibodies that recognize rat macrophages (anti-ED1) and T lymphocytes (anti-CD43). The analysis showed significant increase in the infiltration of macrophages predominantly in the outer medulla 24 h after glycerol. The macrophage infiltration was not affected by silymarin. The T lymphocytes infiltration was not significantly increased 24 h after glycerol injection in both groups ().
TABLE 2. Leukocytes staining in the renal tissue (immunohistochemistry) 24 h after glycerol
Renal expression of apoptosis regulating proteins
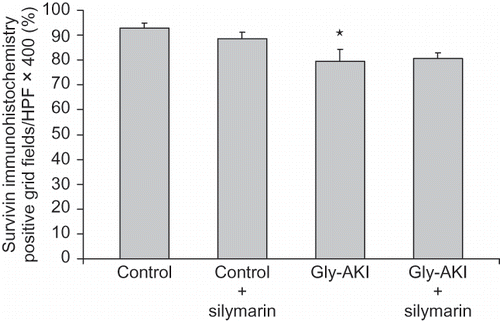
Western blot analysis of proteins extracted from the outer medulla 6 h after glycerol injection showed increased expression of total p53 in Gly-AKI rats treated with silymarin when compared with the controls and untreated rats (A). More importantly, the expression of phosphorylated p53 (p-p53) protein that is the functional active form was increased in Gly-AKI rats, mainly in silymarin-treated animals (B). The p53 transcriptional regulated proapoptotic Bax protein followed a similar pattern of expression, being overexpressed in Gly-AKI + silymarin treated rats (). On the contrary, the expression of the anti-apoptotic Bcl-2 was abrogated in Gly-AKI rats treated with silymarin (). The immunohistochemistry analysis of the survivin expression in the renal tissue showed that the protein is strongly expressed in tubular cells of the renal cortex and outer medulla in healthy control rats and that this expression is slightly reduced 6 h after glycerol injection (). The quantitative analysis showed that 93% of the cortical grid fields/HPF were positive for survivin in the control rats whereas this percentage declined to 79% in Gly-AKI (p =0.05). The treatment with silymarin did not affect the survivin expression in controls or Gly-AKI rats (88.5 and 80.5% of positive grid fields/HPF).
FIGURE 6. Renal expression of the p53 protein (Western blot) 6 h after glycerol injection. (A) There was strong expression of total p53 in all groups and it was significantly exacerbated in Gly-AKI rats treated with silymarin. (B) Phosphorylated p53 (p-p53) expression was very weak in controls and significantly enhanced in Gly-AKI rats. Each band corresponds to a distinct rat and 2 bands/group are shown that are representative of 2–4 rats/group. Densitometry analysis was normalized by β-actin hybridization.
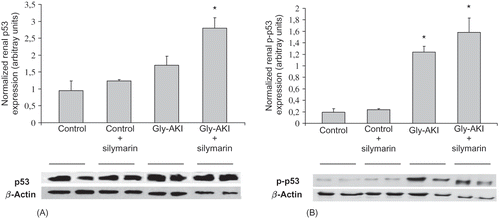
FIGURE 7. Renal expression of the Bax protein (Western blot) 6 h after glycerol injection. There was weak expression of the proapoptotic Bax protein in controls and Gly-AKI + vehicle. The Bax expression corrected by β-actin hybridization was significantly enhanced in Gly-AKI + silymarin rats. Each band corresponds to a distinct rat and 2 bands/group are shown that are representative of 2–4 rats/group.
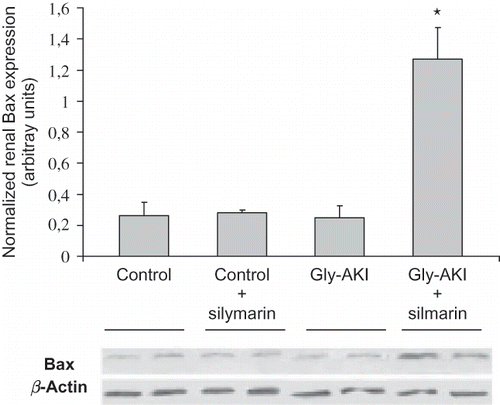
FIGURE 8. Renal expression of the Bcl-2 protein (Western blot) 6 h after glycerol injection. The expression of anti-apoptotic Bcl-2 protein was detected in controls and Gly-AKI + vehicle and was so tenuous in Gly-AKI + silymarin rats that could not be detected by the densitometry software. Each band corresponds to a distinct rat and 2 bands/group are shown that are representative of 2–4 rats/group.
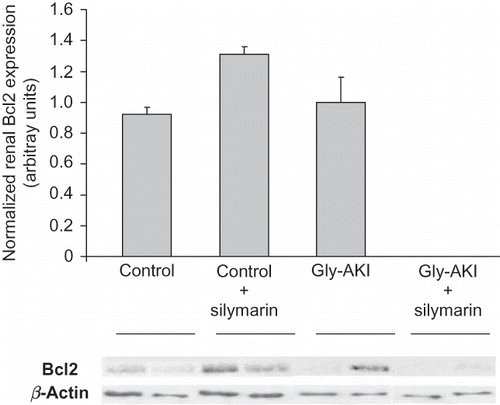
FIGURE 9. Renal expression of survivin (immunohistochemistry) 6 h after glycerol injection. The tubular expression analyzed through quantification of the number of cortical positive grid fields/HPF X400 was more intense in the deep cortex and outer medulla. Control rats showed stronger surviving expression than Gly-AKI. Silymarin treatment did not reduce survivin expression in Gly-AKI. n = 4–6 rat/group.
DISCUSSION
The administration of silymarin aggravated the renal functional impairment 24 h after glycerol injection. The treatment was unable to reduce the lipid and DNA oxidative stress estimated by MDA and 8-OHdG, respectively. Silymarin increased the renal tubular apoptosis after glycerol injection, particularly in the outer medulla, and had no effect in the magnitude of tubular necrosis or in the renal leukocyte infiltration at this point. We have shown previously that attenuation of apoptosis using pan-caspase and caspase-3 inhibitors reduced the renal impairment in Gly-AKICitation15 and, in a subsequent study, that hepatocyte growth factor (HGF) blockade increased the tubular apoptosis and the renal impairment in the same model.Citation27 However, these maneuvers also interfered with the renal inflammatory response and the specific role of apoptosis in the functional response could not be ascertained. In this study, silymarin specifically exacerbated the tubular apoptosis that resulted in aggravation of the renal impairment in these rats. These data support a central role for tubular apoptosis in the development of Gly-AKI in rats.
Excessive tubular reactive oxygen species (ROS) generation may activate numerous protein kinases (e.g., p38, ataxia telangiectasia mutated (ATM) kinase) that phosphorylate and stabilize p53.Citation28–30 The phosphorylation of serine-20 residue in p53 interfere with p53’s association with its inhibitor, Mdm2, reducing p53 degradation.Citation31 ROS induces DNA damage, which is a robust trigger of p53 activation.Citation32 Furthermore, increased p53 expression has been observed in different cell cultures exposed to silymarin.Citation6–9 We observed (B) a trend toward increase in 8-OHdG expression in control rats treated with silymarin compared with control rats treated with vehicle, suggesting an intrinsic genotoxic effect of silymarin. The persistence of the oxidative stress and the additional silymarin-induced p53 activation resulted in increased p53 expression and activation in Gly-AKI rats treated with silymarin.
The p53 is a critical activator of the intrinsic pathway of apoptosis. A large and growing number of p53 target genes have been implicated in its apoptotic effects. These include transcriptional activation of the proapoptotic Bax protein (Bcl-2 family member)Citation33 and proapoptotic BH3-only proteins (NOXA, PUMA), Apaf-1, and p53-regulated-apoptosis-inducing protein 1(p53AIP1).Citation34–36 p53 represses expression of the prosurvival protein Bcl-2Citation28 and of the inhibitor of apoptosis protein (IAP) survivin.Citation37,Citation38 The activation of BH3-only proteins leads to Bax activation, which induces permeabilization of the outer mitochondrial membrane and release of proapoptotic factors such as cytochrome c, SMAC/DIABLO, and apoptosis inducing factor (AIF). The opposite regulation of mitochondrial permeability is exerted by Bcl-2 and BclxL proteins. The cytochrome c released binds to Apaf-1 and caspase-9 in the apoptosome, resulting in the activation of the caspase-9 that cleaves and activates the effector caspase-3 and caspase-7. IAPs, for example, survivin, may intercept apoptosis through the inhibition of apoptosome formation and also may directly inhibit the action of caspase-9 and effector caspase-3 and caspase-7.
In this study, Gly-AKI rats treated with silymarin showed increased expression of the proapoptotic Bax protein and reduced expression of anti-apoptotic Bcl-2 protein. The activation of the intrinsic apoptosis cascade resulted in great expression of cleaved caspase-3 in tubular cells. A similar pattern of p53-dependent apoptosis had already been related by Katiyar et al.Citation39 in preneoplastic epidermal cell line JB6 C141 treated with silymarin. We further examined the renal expression of the IAP-survivin in our experimental condition. Initially, it was believed that survivin was expressed only in immature tissues during embryogenesis or in tumoral tissues. Recently, Lechler et al.Citation40 showed unequivocally high survivin expression in the proximal tubules of healthy adult rats, mice, and human kidneys. They also showed reduction in the survivin expression after hypoxic insult to tubular cells in vitro. In agreement with their data, we observed a strong tubular expression of survivin in healthy control rats, predominantly in the renal cortex (). Furthermore, a slight but significant reduction on its renal expression was observed 6 h after glycerol injection. These data suggest that survivin downregulation may play a role in the development of tubular apoptosis in Gly-AKI. Although previous studies showed that administration of silymarin reduced survivin expression in vitro,Citation7 we did not observe additional downregulation on its expression in Gly-AKI rats treated with silymarin.
Our data contrast with the previous studies that showed that silymarin reduced the oxidative stress and tissue damage in rats with toxic hepatitis, cardiac ischemia/reperfusion injury, and renal ischemia/reperfusion injury.Citation2,Citation3,Citation18,Citation19,Citation41 The discrepant effect on oxidative stress suggests that heme-catalyzed reactive species generation may differ from reactive species generated in other experimental conditions. Alternatively, silymarin-induced p53 activation could upregulate genes that encode ROS-generating enzymes (PIG 3, PIG 6)Citation42 or suppress antioxidant genes (MnSOD),Citation43 either way tipping the balance toward oxidative stress. Furthermore, Gly-AKI rats treated with silymarin showed increased p53-mediated tubular apoptosis and exacerbation of the renal function impairment 24 h after glycerol injection. Our data, derived from Gly-AKI study in rats, indicate that silymarin is not a good candidate for prevention of AKI after rhabdomyolysis.
Acknowledgments
This work was supported by grant from Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP).
REFERENCES
- Kroll DJ, Shaw HS, Oberlies NH. Milk thistle nomenclature: Why it matters in cancer research and pharmacokinetic studies. Integr Cancer Ther. 2007;6:110–119.
- Mansour HH, Hafez HF, Fahmy NM. Silymarin modulates cisplatin-induced oxidative stress and hepatotoxicity in rats. J Biochem Mol Biol. 2006;39:656–661.
- Pradeep K, Mohan CV, Gobianand K, Karthikeyan S. Silymarin modulates the oxidant-antioxidant imbalance during diethyonitrosinamine induced oxidative stress in rats. Eur J Pharmacol. 2007;560:110–116.
- Comelli MC, Mengs U, Schneider C, Prosdomici M. Toward definition of the mechanism of action of silymarin: Activities related to cellular protection from toxic damage induced by chemotherapy. Integr Cancer Ther. 2007;6:120–139.
- Singh RP, Gu M, Agarwal R. Silibinin inhibits colorectal growth by inhibiting tumor cell proliferation and angiogenesis. Cancer Res. 2008;68:2043–2050.
- Tyagi A, Singh RP, Agarwal C, Agarwal R. Silibinin activates p53-caspase 2 pathway and causes caspase-mediated cleavage of Cip/p21 in apoptosis induction in bladder transitional-cell papilloma RT4 cells: Evidence for a regulatory loop between p53 and caspase 2. Carcinogenesis. 2006;27:2269–2280.
- Singh RP, Dhanalakshmi S, Agarwal C, Agarwal R. Silibinin strongly inhibits growth of human endothelial cells via cell cycle arrest and downregulation of survivin, Akt and NF-kappaB: Implications for angioprevention and antiangiogenic therapy. Oncogene. 2005;24:1188–1202.
- Dhanalaklshmi S, Agarwal C, Singh RP, Agarwal R. Silibinin up-regulates DNA-protein kinase-dependent p53 activation to enhance UVB-induced apoptosis in mouse epithelial JB6 cells. J Biol Chem. 2005;280:20375–20383.
- Mallikarjuna G, Dhanalaklshmi S, Singh RP, Agarwal C, Agarwal R. Silibinin protects against photocarcinogenesis via modulation of cell cycle regulators, mitogen-activated protein kinases, and Akt signaling. Cancer Res. 2004;64:6349–6356.
- Paller MS. Hemoglobin and myoglobin induced acute renal failure in rats: Role of iron in nephrotoxicity. Am J Physiol. 1988;255:F539–F544.
- Moore KP, Holt GS, Patel RP, A causative role for redox cycling of myoglobin and its inhibition by alkalinization in the pathogenesis and treatment of rhabdomyolysis-induced renal failure. J Biol Chem. 1998;273:31731–31737.
- Zager RA, Burkhart KM, Conrad DS, Gmur DJ. Iron, heme oxygenase and glutathione: Effects on myohemoglobinuric proximal tubular injury. Kidney Int. 1995;48:1624–1634.
- Shah SV, Walker PD. Evidence suggesting a role for hydroxyl radical in glycerol-induced acute renal failure. Am J Physiol. 1988;255:F438–F443.
- Guidet B, Shah SV. Enhanced in vivo H2O2 generation by rat kidney in glycerol-induced renal failure. Am J Physiol. 1989;257:F440–F445.
- Homsi E, Janino P, de Faria JB. Role of caspases on cell death, inflammation, and cell cycle in glycerol-induced acute renal failure. Kidney Int. 2006;69:1385–1392.
- Shulman LM, Yuhas Y, Folkis I, Gavendo S, Knecht A, Eliahou HE. Glycerol-induced acute renal failure is mediated by tumor necrosis factor. Kidney Int. 1993;43:1397–1401.
- Wolfert AI, Oken DE. Glomerular hemodynamics in established glycerol-induced acute renal failure in the rat. J Clin Invest. 1989;84:1967–1973.
- Turgut F, Bayrak O, Catal F, Antioxidant and protective effects of silymarin on ischemia and reperfusion injury in the kidney of rats. Int Urol Nephrol. 2008;40:453–460.
- Senturk H, Kabay S, Bayramoglu G, Silymarin attenuates the renal ischemia/reperfusion injury-induced morphological changes in the rat kidney. World J Urol. 2008;26:401–407.
- Manna SK, Mukhopadhyay A, Van NT, Aggarwal BB. Silymarin suppresses TNF-induced activation of NF-kappa B, c-Jun N-terminal kinase, and apoptosis. J Immunol. 1999;163:6800–6809.
- Mansour HH, Hafez HF, Fahmy HM. Silymarin modulates cisplatin-induced oxidative stress and hepatotoxicity in rats. J Biochem Mol Biol. 2006;39:656–661.
- Schuman J, Prockl J, Kiemer AK, Vollmar AM, Bang R, Tiegs G. Silibilin protects mice fom T cell-dependent liver injury. J Hepatol. 2003;39:333–340.
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1079;95:351–358.
- Krajewska M, Smith LH, Rong J, Image analysis algorithms for immunohistochemical assessment of cell death events and fibrosis in tissue sections. J Histochem Cytochem. 2009;57:649–663.
- Gupta A, Berg DT, Gerlitz B, Role of protein C in renal dysfunction after polymicrobial sepsis. J Am Soc Nephrol. 2007;18:860–867.
- Duan WR, Garner DS, Williams SD, Funckes-Shippy CL, Spath IS, Blomme EA. Comparison of immunohistochemistry for activated caspase-3 and cleaved cytokeratin 18 with TUNEL method for quantification of apoptosis in histological sections of PC-3 subcutaneous xenografts. J Pathol. 2003;199:221–228.
- Homsi E, Janino P, Amano M, Camara NOS. Endogenous hepatocyte growth factor attenuates inflammatory response in glycerol-induced acute kidney injury. Am J Nephrol. 2009;29:283–290.
- Bulavin DV, Saito S, Hollander MC, Phosphorylation of human p53 by p38 kinase coordinates N-terminal phosphorylation and apoptosis in response to UV radiation. EMBO J. 1999;18:6845–6854.
- Xie S, Wang Q, Wu H, Reactive oxygen species induced-phosphorylation of p53 on serine 20 is mediated in part by PIk3. J Biol Chem. 2001;276:36194–36199.
- Hirao A, Kong YY, Matsuoka S, DNA damage-induced activation of p53 by the checkpoint kinase (Chk2). Science. 2000;287:1824–1827.
- Fang S, Jensen JP, Ludwig RL, Vousden KH, Weissman AM. Mdm2 is a RING finger-dependent ubiquitin protein ligase for itself and p53. J Biol Chem. 2000;275:8945–8951.
- Martindale JL, Holbrook NJ. Cellular response to oxidative stress: Signaling for suicide and survival. J Cell Physiol. 2002;192:1–5.
- Miyashita T, Krajewski S, Krajewska M, Tumor suppressor 53 is a regulator of bcl-2 and bax gene expression in vitro and in vivo. Oncogene. 1994;9:1799–1805.
- Nakano K, Vousden KH. PUMA, a novel proapoptotic gene,is induced by p53. Mol Cell. 2001;7:683–694.
- Oda E, Ohki R, Murasawa H, Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science. 2000;288:1053–1058.
- Oda K, Arakawa H, Tanaka T, p53AIP1, a potential mediator of p53-dependent apoptosis, and its regulation by Ser-46-phosphorylated p53. Cell. 2000;102:849–862.
- Mirza A, McGuirk TN, Hockenberry Q, Wu H, Ashar S, Black S. Human survivin is negatively regulated by wild type p53 and participate in p53-dependent apoptotic pathway. Oncogene. 2002;2:2613–2622.
- Hoffman WH, Biade JT, Zilfou J, Chen J, Murphy M. Transcription repression of the anti-apoptotic survivin gene by wild type p53. J Biol Chem. 2002;277:3247–3257.
- Katiyar SK, Roy AM, Baliga MS. Silymarin induces apoptosis primarily through a p53-dependent pathway involving Bcl-2/Bax, cytochrome c release, and caspase activation. Mol Cancer Ther. 2005;4:207–216.
- Lechler P, Wu X, Bernhardt W, Campean V, The tumor gene survivin is highly expressed in adult tubular cells. Implications for a pathophysiological role in the kidney. Am J Pathol. 2007;171:1483–1489.
- Rao PR, Viswanath RK. Cardioprotective activity of silymarin in ischemia-reperfusion-induced myocardial infarction in albino rats. Exp Clin Cardiol. 2007;12:179–187.
- Polyak K, Xia Y, Zweier JL, Kinzler KW, Vogelstein B. A model for p53-induced apoptosis. Nature. 1997;389:300–395.
- Drane P, Bravard A, Bouvard V, May E. Reciprocal down-regulation of p53 and SOD2 gene expression-implication in p53 mediated apoptosis. Oncogene. 2001;20:430–439.