Abstract
Introduction: The aim of this study was to evaluate lipid peroxidation (LP) and free radical scavenging enzyme activities in kidney tissue of vitamin B6-deficient rats. Material and Methods: The rats were divided into control and vitamin B6-deficient groups. After 4 weeks of feeding, animals in all groups were anesthetized by thiopental sodium (50 mg/kg). Thoraces were opened, 2 mL blood samples were taken from aortas, then the rats were killed by cervical dislocation, and kidney tissues were removed. Biochemical measurements in kidney tissue were carried out using a spectrophotometer. Results: Total superoxide scavenger activity (TSSA), nonenzymatic superoxide scavenger activity (NSSA), superoxide dismutase (SOD) activities, and antioxidant potential (AOP) values in the vitamin B6-deficient group were significantly lower than those of the control group, whereas glutathione peroxidase (GSH-Px), glutathione reductase (GRD), glutathione-S-transferase (GST) activities, and malondialdehyde (MDA) level were significantly higher than those of the control group (p < 0.05). Discussion: The results show that vitamin B6 deficiency causes an attenuation in antioxidant defense system and an increase in oxidative stress in kidney tissue of rats.
INTRODUCTION
Oxygen free radicals (OFRs), such as superoxide radical () and hydroxyl radical (OH•–), are highly reactive species generated by biochemical redox reactions as part of normal cell metabolism. Oxygen metabolism in aerobic organisms has some advantages. In view of the generation of OFR, certain adverse effects also occur. Practically, all the essential biomolecules can undergo oxidative reactions mediated by OFR. The study of OFR and their proposed effects on biological systems has become an important area of biomedical research in recent years.Citation1–3
Vitamin B6 has been shown to be important for normal cognitive function and in lowering the incidence of coronary heart disease among the elderly.Citation4–6 In addition, vitamin B6 supplementation has been shown to reduce diabetic complications and incidences of neurodegenerative diseases in varying degrees.Citation4–8 Vitamin B6 refers to three primary forms of water-soluble vitamins: pyridoxine, pyridoxal phosphate, and pyridoxamine. Some studies have reported antioxidant activities of vitamin B6.Citation9,Citation10 Kannan and Jain reported that pyridoxine and pyridoxamine inhibit generation, reduce lipid peroxidation (LP), and prevent damage to mitochondrial membrane integrity in U937 cells.Citation8 Vitamin B6 deficiency affects lipid metabolism, modifying fatty acid composition of some tissues and increasing plasma triglyceride and cholesterol levels. LP has been linked with changed membrane structure and enzyme inactivation. The induction of LP is considered to be important in the etiology of many diseases.Citation11–15
Cells have different antioxidant systems and various antioxidant enzymes to defend themselves against free radical attacks. Superoxide dismutase (SOD), the first line of defense against OFR, catalyzes the dismutation of into hydrogen peroxide (H2O2). Glutathione (GSH)-dependent antioxidant system consisting of reduced GSH and an array of functionally related enzymes plays a fundamental role in cellular defense against reactive free radicals and other oxidant species. Of these enzymes, glutathione peroxidase (GSH-Px) is a selenoprotein that reduces hydroperoxides as well as H2O2 but oxidizing GSH. A number of potentially toxic electrophilic xenobiotics (such as certain carcinogens, bromobenzene and chlorobenzene) are conjugated to the nucleophilic GSH by glutathione-S-transferases (GSTs) present in high amounts in cell cytosol. GST can also catalyze reactions reducing peroxides like GSH-Px. Reduction of oxidized glutathione (GSSG) to GSH is mediated by the widely distributed enzyme GSH reductase (GRD) that uses NADPH as the reducing agent.Citation16–18
Chronic renal failure and vitamin B6 deficiency share some common features such as peripheral neuropathy, normochromic anemia, depression of the immune system disturbances, improvement with low-protein diet, and increased body oxalate levels.Citation19
To our knowledge, there is no study that simultaneously investigates antioxidant potential (AOP), total (enzymatic plus nonenzymatic) superoxide scavenger activity (TSSA), nonenzymatic superoxide scavenger activity (NSSA), GSH-Px, GRD, GST, catalase (CAT), SOD activities, and malondialdehyde (MDA) levels in kidney tissue in vitamin B6-deficient rats and control group. Therefore, in this study, we aimed to investigate the effects on LP and free radical scavenging enzyme activities in kidney tissue of vitamin B6-deficient rats.
MATERIALS AND METHODS
Twenty male rats (4 weeks old, Sprague–Dawley strain) with a weight of 50–60 g were used for the experiment. The animals were randomly divided into two groups of 10 rats each. All animals received humane care in compliance with the guidelines of Ataturk University Research Council's criteria. The composition of the diet was as described in .Citation11
TABLE 1. The composition of the experimental diet
Biochemical analyses
After 4 weeks of feeding, animals in all groups were anesthetized by thiopental sodium (50 mg/kg). Thoraces were opened, 2 mL blood samples were taken from aortas, then the rats were killed by cervical dislocation, and kidney tissues were removed, washed out from contaminated blood with cold water, and homogenized in 10-fold physiological saline solution by using a homogenizer (Omni Accessory Pack International homogenizer, Warrenton, Virginia, USA). The homogenate was centrifuged at 10,000 × g for 1 h to remove debris. The supernatant was collected and all assays were carried out on this fraction.
The kidney tissue AOPs were measured by a method described by Durak et al.Citation20 According to this method, in the reaction medium enriched with fish oil, samples (supernatant obtained after centrifugation) were exposed to produced by the xanthine–xanthine oxidase system for 1 h. Fish oil was used for this purpose because it is a polyunsaturated oil, which is very sensitive to free radical attack. As known, when there is an inability in the cell to eliminate free radicals, unsaturated free fatty acids would be easily oxidized and then the MDA concentration would be increased. By using this reaction system, it is possible to obtain more precise information about AOP of the samples. MDA levels were measured in the control and sample studies. AOP values were assessed from the difference between MDA levels of control and sample studies. AOP values were expressed as nmol/mg protein.h−1. The kidney tissue MDA levels were measured by the spectrophotometric method of Ohkawa et al.Citation21 Total thiobarbituric acid-reactive substances were expressed as MDA. MDA levels are also expressed as nmol/mg protein.
TSSA, NSSA, and SOD assays were performed in the samples before and after trichloroacetic acid (TCA) 20% (w/v).Citation22 GSH-Px, GRD, and GST activities were measured as described, respectively.Citation23–25 Protein content was determined by using the Bradford method.Citation26 Results were expressed in U/mg protein for TSSA, NSSA, SOD, GRD, and GST activities; mIU/mg protein for GSH-Px activity; and nmol/mg protein for MDA levels. One unit of TSSA, NSSA, and SOD was defined as the amount of enzyme protein causing 50% inhibition in nitroblue tetrazolium reduction rate. Biochemical measurements were carried out using a spectrophotometer (CECIL CE 3041, Cambridge, UK). Plasma pyridoxal-5-phosphate levels were measured by high-performance liquid chromatography.
Statistical analysis
The findings were expressed as mean ± SD. Statistical and correlation analyses were undertaken using the Mann–Whitney U-test and Spearman's rank correlation test, respectively. A p-value < 0.05 was accepted as statistically significant. Statistical analysis was performed with Statistical Package for the Social Sciences for Windows (SPSS, version 11.0, Chicago, Illinois, USA).
RESULTS
All parameters are shown in . As seen from the table, kidney tissue TSSA, NSSA, SOD activities, AOP, and plasma pyridoxal-5-phosphate levels in the vitamin B6-deficient group were significantly lower than those of the control group (p < 0.05), whereas GSH-Px, GST, GRD activities, and MDA levels were significantly higher than those of the control group (p < 0.05).
TABLE 2. Comparison of different variables between the study and control groups
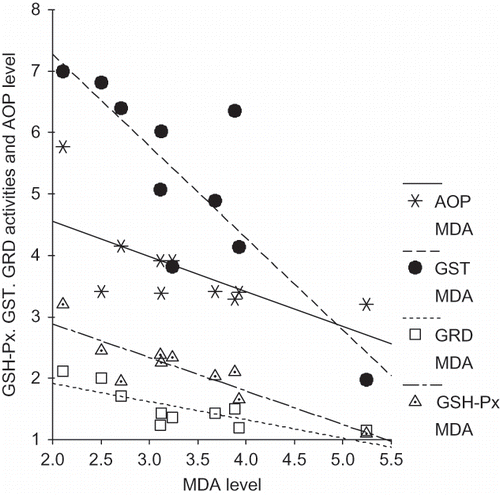
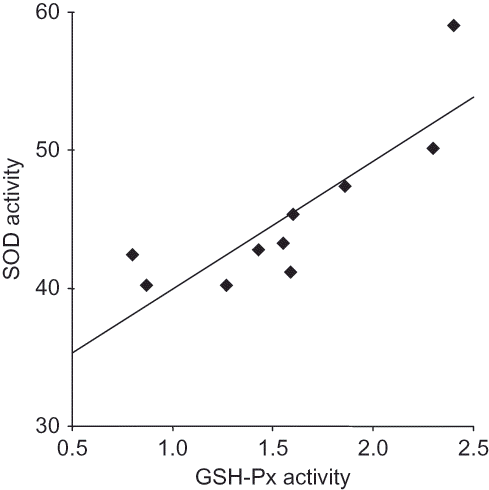
Correlation analysis revealed significant negative correlations between MDA and GST (r = –0.82, p < 0.005), MDA and GRD (r = –0.77, p < 0.01), MDA and SOD (r = –0.79, p < 0.01), and MDA and AOP (r = –0.79, p < 0.01) in the vitamin B6-deficient group (). There was a significant positive correlation between GSH-Px and SOD (r = 0.87, p < 0.001) in the control group (). However, no correlation between the groups in same parameters could be found.
DISCUSSION
Oxidative stress is an imbalance between the production of free radicals that contain unpaired electrons and the antioxidant defenses buffering the oxidative damages. Oxidative effects of free radicals are controlled by exogenous antioxidants, such as vitamins E and C, and also by endogenous antioxidants. Under some conditions, increases in oxidants and decreases in antioxidants cannot be prevented, and oxidative–antioxidative balance shifts toward the oxidative stress.Citation27
In this study, determination of some oxidative stress parameters has been carried out in the kidney tissue of vitamin B6-deficient rats. The data show that kidney tissue TSSA, NSSA, SOD activities, and AOP values are decreased; GSH-Px, GST, GRD activities, and MDA level are increased in vitamin B6-deficient rats. These results explicitly indicate that vitamin B6 deficiency causes a decrease in antioxidant defense system and an increase in oxidant stress in kidney tissue in rats.
Reactive oxygen species (ROS) have the potential to cause reversible or irreversible damage in all kinds of biochemical materials containing nucleic acids, proteins, free amino acids, lipids, lipoproteins, carbohydrates, and connective tissue macromolecules. Biological membranes and intracellular components, which are rich in polyunsaturated fatty acid, can be easily affected by free radicals. One of the primary events in oxidative cellular damage is the oxidation of membrane lipids. Measurement of the breakdown products such as MDA, a highly toxic molecule, which is used as a biological marker of oxidative stress, is the most common approach to determine the degree of LP induced by ROS.Citation11 Increased kidney tissue MDA levels in vitamin B6-deficient rats of our study were in concordance with previous reports.Citation28,Citation29 This confirms the presence of increased oxidative stress in vitamin B6-deficient rats.
The findings showed increased GSH-Px and decreased SOD activities in kidney tissue. Alterations in the antioxidant enzymes in kidney tissue of rats fed with vitamin B6-deficient diet are inconsistent. SOD dismutates to H2O2. Although H2O2 is weakly reactive, its major toxicity derives from its conversion to the highly toxic OH•– through the Fenton or Haber–Weiss reactions. GSH-Px detoxifies H2O2 by converting it into water and molecular oxygen. It seems that increased
radical is not converted to H2O2 because of decreased activity of SOD. Positive correlation between SOD and GSH-Px in the control group may suggest collaboration present between these two antioxidant enzymes (). However, this collaboration seems to be impaired in the vitamin B6-deficient group. These antioxidant enzymes protect the cell constituents from damage by OFRs.Citation11,Citation14
The antioxidant status (composed of enzymatic and nonenzymatic antioxidants) is known to be a barrier (both endogenous and exogenous) against free radical attacks in all body compartments.Citation26 In this study, we found that TSSA, NSSA, and AOP level in kidney tissue of rats fed with vitamin B6-deficient diet were significantly decreased compared with the control group. AOP reflects the total capacity of the enzymatic and nonenzymatic antioxidant systems. Weakness of AOP indicates impairment in the total defense system. When SOD activity and TSSA are suppressed by increasing oxidant stress in vitamin B6 deficiency, radicals may be elevated in the kidney tissues. SOD enzyme is the most important defense mechanism against the
radicals produced in the cells.Citation30 Increased
radicals might be responsible for the oxidative damage reflected as increased kidney tissue MDA levels in vitamin B6-deficient group in our study. Increased GSH-Px activities might be an attempt to lower H2O2, a potent toxic metabolite for living cells.Citation30
GSH synthesis depends on the availability of cysteine, which is synthesized by vitamin B6-dependent enzymes cystathionine β-synthase and cystathionine γ-lyase from methionine. It has been reported that cysteine synthesis reduced in vitamin B6-deficient rats because of the altered activity of these enzymes, which leads to decreased GSH synthesis.Citation11,Citation30–32 It might be concluded that increased GRD and GST activities in vitamin B6-deficient rats in this study were related to decreased synthesis of GSH, which has been mentioned above, because of increased oxidative stress in response to vitamin B6 deficiency. GST utilizes GSH for detoxification of a number of xenobiotics whereas reduction of GSSG to GSH is mediated by the widely distributed enzyme GRD.
How vitamin B6 compounds scavenge OFRs and thereby inhibit LP reaction has not been clearly understood yet. However, the potency of a chemical to act as an antioxidant is generally determined by several factors, such as intrinsic chemical reactivity of the antioxidant toward the radical. The phenolic compounds have high reactivity toward the peroxy radical, because a phenolic group can react much faster with the peroxy radical than peroxy radical can react with lipid in the membrane. This reactivity is determined by the bond dissociation energy, such as that of the O–H bond in case of phenolic group, as well as the resonance stabilization of the resulting radical, the redox potential, and the steric hindrance to abstraction of the hydroxyl or amine hydrogen by peroxy radicals. Functional groups such as hydroxyl and amine can generally scavenge oxygen radicals.Citation8,Citation33 Vitamin B6 groups of compounds have both the hydroxyl and amine groups' substitution on a pyridine ring; those groups can scavenge OFRs. This condition may explain, at least in part, the scavenging action of vitamin B6 compounds. Thus, besides the scavenging function of vitamin B6, involvement of other biochemical pathways cannot be ruled out in explaining the mechanism of action of these vitamin compounds.Citation8
It has been suggested in this study that vitamin B6 deficiency disturbs nutrition balance and causes oxidant stress and LP by means of excessive free radical production in kidney tissue in rats.
Declaration of interest: None of the authors has a commercial interest, financial interest, and/or other relationship with manufacturers of pharmaceuticals, laboratory supplies, and/or medical devices or with commercial providers of medically related services.
REFERENCES
- Kaya H, Taysi S, Kaya A, Investigation of free radical scavenging enzyme activities and lipid peroxidation in liver tissue of zinc deficient rats. Asian J Chem. 2008;20:1068–1074.
- Aktan B, Taysi S, Gumustekin K, Bakan N, Sutbeyaz Y. Evaluation of oxidative stress in erythrocytes of guinea pigs with experimental otitis media and effusion. Ann Clin Lab Sci. 2003;33:232–236.
- Memisogullari R, Taysi S, Bakan E, Capoglu I. Antioxidant status and lipid peroxidation in type II diabetes mellitus. Cell Biochem Funct. 2003;21:291–296.
- Bryan J, Calvaresi E, Hughes D. Short-term folate, vitamin B-12 or vitamin B-6 supplementation slightly affects memory performance but not mood in women of various ages. J Nutr. 2002;132:1345–1356.
- Calvaresi E, Bryan J. B vitamins, cognition, and aging: A review. J Gerontol B Psychol Sci Soc Sci. 2001;56:327–339.
- Fletcher RH, Fairfield KM. Vitamins for chronic disease prevention in adults: Clinical applications. JAMA. 2002;287:3127–3129.
- Fairfield KM, Fletcher RH. Vitamins for chronic disease prevention in adults: Scientific review. JAMA. 2002;287:3116–3126.
- Kannan K, Jain SK. Effect of vitamin B6 on oxygen radicals, mitochondrial membrane potential, and lipid peroxidation in H2O2-treated U937 monocytes. Free Radic Biol Med. 2004;36:423–428.
- Endo N, Nishiyama K, Otsuka A, Kanouchi H, Taga M, Oka T. Antioxidant activity of vitamin B6 delays homocysteine-induced atherosclerosis in rats. Br J Nutr. 2006;95:1088–1093.
- Stocker P, Lesgards JF, Vidal N, Chalier F, Prost M. ESR study of a biological assay on whole blood: Antioxidant efficiency of various vitamins. Biochim Biophys Acta. 2003;1621:1–8.
- Taysi S. Oxidant/antioxidant status in liver tissue of vitamin B6 deficient rats. Clin Nutr. 2005;24:385–389.
- Cabrini L, Bergami R, Fiorentini D, Marchetti M, Landi L, Tolomelli B. Vitamin B6 deficiency affects antioxidant defences in rat liver and heart. Biochem Mol Biol Int. 1998;46:689–697.
- Selvam R, Ravichandran V. Effect of oral methionine and vitamin E on blood lipid peroxidation in vitamin B6 deficient rats. Biochem Int. 1991;23:1007–1017.
- Taysi S, Polat F, Gul M, Sari RA, Bakan E. Lipid peroxidation, some extracellular antioxidants, and antioxidant enzymes in serum of patients with rheumatoid arthritis. Rheumatol Int. 2002;21:200–204.
- Taysi S, Gul M, Sari RA, Akcay F, Bakan N. Serum oxidant/antioxidant status of patients with systemic lupus erythematosus. Clin Chem Lab Med. 2002;40:684–688.
- Gul M, Demircan B, Taysi S, Effects of endurance training and acute exhaustive exercise on antioxidant defense mechanisms in rat heart. Comp Biochem Physiol A Mol Integr Physiol. 2006;143:239–245.
- Oztasan N, Taysi S, Gumustekin K, Endurance training attenuates exercise-induced oxidative stress in erythrocytes in rat. Eur J Appl Physiol. 2004;91:622–627.
- Taysi S, Cikman O, Kaya A, Increased oxidant stress and decreased antioxidant status in erythrocytes of rats fed with zinc-deficient diet. Biol Trace Elem Res. 2008;123:161–167.
- Chazot C, Kopple JD. Vitamin metabolism and requirements in renal disease and renal failure. In: Kopple JD, Massry SG, eds. Kopple and Massry's Nutritional Management of Renal Disease. Philadelphia: Lippincott Williams &Wilkins; 2003:415–478.
- Durak I, Karabacak HI, Buyukkocak S, Impaired antioxidant defense system in the kidney tissues from rabbits treated with cyclosporine. Protective effects of vitamins E and C. Nephron. 1998;78:207–211.
- Ohkawa H, Ohishi N, Yagi K. Reaction of linoleic acid hydroperoxide with thiobarbituric acid. J Lipid Res. 1978;19:1053–1057.
- Durak I, Canbolat O, Kacmaz M, Ozgen G, Ozturk HS. Antioxidant interferences in superoxide dismutase activity methods using superoxide radical as substrate. Clin Chem Lab Med. 1998;36:407–408.
- Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70:158–169.
- Pinto RE, Bartley W. The effect of age and sex on glutathione reductase and glutathione peroxidase activities and on aerobic glutathione oxidation in rat liver homogenates. Biochem J. 1969;112:109–115.
- Sen CK, Marin E, Kretzschmar M, Hanninen O. Skeletal muscle and liver glutathione homeostasis in response to training, exercise, and immobilization. J Appl Physiol. 1992;73:1265–1272.
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254.
- Demirbag R, Yilmaz R, Guzel S, Celik H, Kocyigit A, Ozcan E. Effects of treadmill exercise test on oxidative/antioxidative parameters and DNA damage. Anadolu Kardiyol Derg. 2006;6:135–140.
- Selvam R, Ravichandran V. Restoration of tissue antioxidants and prevention of renal stone deposition in vitamin B6 deficient rats fed with vitamin E or methionine. Indian J Exp Biol. 1993;31:882–887.
- Benderitter M, Hadj-Saad F, Lhuissier M, Maupoil V, Guilland JC, Rochette L. Effects of exhaustive exercise and vitamin B6 deficiency on free radical oxidative process in male trained rats. Free Radic Biol Med. 1996;21:541–549.
- Biri A, Kavutcu M, Bozkurt N, Devrim E, Nurlu N, Durak I. Investigation of free radical scavenging enzyme activities and lipid peroxidation in human placental tissues with miscarriage. J Soc Gynecol Investig. 2006;13:384–388.
- Guilland JC, Bereksi-Reguig B, Lequeu B, Moreau D, Klepping J, Richard D. Evaluation of pyridoxine intake and pyridoxine status among aged institutionalized people. Int J Vitam Nutr Res. 1984;54:185–193.
- Hoyumpa AM. Mechanisms of vitamin deficiencies in alcoholism. Alcohol Clin Exp Res. 1986;10:573–581.
- Noguchi N, Okimoto Y, Cynshi O, Kodama T, Niki E. Inhibition of oxidative modification of low density lipoprotein by novel antioxidant BO-653 prepared by theoretical design. In: Paker L, Ong ASH, eds. Biological Oxidants and Antioxidants. Champaign: AOCS Press; 1998:139–152.