Abstract
Aim: The aim of this study was to determine the survival of patients treated by peritoneal dialysis (PD) and hemodialysis (HD) and to detect any association with the type of metabolic changes. Methods: The outcome of clinical treatment of 407 dialysis patients was analyzed over a 4-year period. This included the demographic characteristics, the duration of dialysis, smoking, residual renal function, existence of metabolic syndrome and malnutrition, waist girth, body mass index (BMI), comorbidity, and routine biochemical parameters. Results: The overall mortality of the treated patients during the 4-year period was 53%, 37% for HD patients and 65% for PD patients. Metabolic syndrome was the dominant metabolic disorder affecting more than half of the HD patients, as well as being a predictive mortality parameter (β = 0.560; p = 0.045). The PD-treated patients had an equal prevalence of metabolic syndrome and malnutrition, whereas statistically significant predictors of mortality outcome were BMI (β = 0.088; p = 0.002) and waist girth (β = 0.023; p = 0.031). The median survival value was significantly higher for HD patients [108 months; 95% confidence interval (CI) 65–151]. Residual renal function in PD patients was significantly related to mortality (p = 0.045). Conclusion: Metabolic syndrome is a predictive parameter of mortality for HD patients, whereas for PD patients it is the waist girth and BMI. Preserved residual renal function in patients on PD is an important factor in reducing mortality.
INTRODUCTION
In general terms, the mortality of patients, whose level of kidney insufficiency requires treatment by one of the models of dialysis procedures, is extremely high.Citation1 Around two-thirds of all dialysis patients in the United States die during the first 5 years from the commencement of treatment, which is a higher mortality rate than for many malignant diseases.Citation2 During the first 2 years of dialysis treatment, the survival of peritoneal dialysis (PD) patients is significantly longer compared to that of hemodialysis (HD)-treated patients. After that, the outcome for both treatments is similar and so the benefit of PD treatment is annulled after 2.5 years.Citation3
Having in mind the significant incidence of cardiovascular diseases (CVD) among dialysis patients and the assumption that more than 80% of dialysis patients could die because of many other mortality risk factors,Citation4 a serious dilemma exists as to which form of dialysis depuration should be recommended for such patients. Unfortunately, there are very few adequate stratification studies which could give an answer to this problem.
One of the main aims of strategical development in nephrology in the twenty-first century is the prevention of the terminal stadium of kidney insufficiency. An important front is transplantation, which will be the main form of treatment of such patients. Xenotransplantation and cloning using the patient's own somatic cells could represent a significant area of alternative therapy. All these activities would decrease the need for dialysis procedures. It is forecast that in a few decades the number of patients who would need these forms of exchange of kidney function will increase but later decrease. The common conclusion of several studies that attempted to determine the mortality rate of PD and HD patients is that the survival of identical subgroups of treated patients in the first 3–4 years of treatment is almost the same.Citation5
AIM
The aim of this study was to determine the survival rate of patients treated by PD or HD and its relation with the types of metabolic change and to detect predictive mortality parameters.
METHODOLOGY
Patients and study design
This was organized as a follow-up study. The study involved 407 dialysis patients, including 242 (59.5%) males and 165 (40.5%) females. Their average age was 65 ± 12.5 years and the average duration of dialysis was 24 ± 47.3 months. The target group was all PD and HD patients who were treated during the period 2005 to 2008 in the Clinical Center “Kragujevac,” Serbia.
Most patients received HD thrice weekly for 3.5–4 hours, using commercially available dialyzers (F6HPS, F7HPS, Fx80-Fresenius®, Bad Homberg, Germany; 14L, 17L-Gambro®, Lund, Sweden). The range of the blood pump during dialysis was 250–320 mL/min, whereas the flow of dialysis fluid was 500 mL/min.
The PD treatment program encompassed mainly patients who received continuous ambulatory PD, with four to five daily changes, whereas a nonsignificant number of patients were dialyzed using intermittent PD. The achieved daily ultrafiltration for these patients was 1000–1200 mL with commercial PD solutions with glucose monohydrate concentration between 1.36 and 3.86 g/dL.
Clinical and biochemical assessments
Blood for laboratory testing was taken from all patients midweek, before HD or early in the morning from PD patients, before the initial exchange. Hematological analyses were made using the COULTER instrument, by flow cytometry, and other biochemical examinations were performed spectrophotometrically on an Ilab-600 device.
Regarding the demographic–clinical parameters, gender, age, duration of dialysis in months, active smokers, and residual renal function (diuresis above 250 mL) were verified and registered. Any significant comorbid stages, that is, CVD, diabetes mellitus, and peripheral vascular diseases, were noted for all the patients.
Anthropometric measurements included waist girth (cm) and body mass index (BMI) using the quotient of body weight (kg) and the square of body height (m2). Values of BMI below 18 kg/m2 and low concentrations of total protein, albumin, and cholesterol defined undernourished patients (malnutrition), whereas patients with BMI above 25 kg/m2 were considered obese. Metabolic syndrome was determined based on at least three criteria proposed by the National Cholesterol Education Program.Citation6 Lethal outcomes were registered, with the determined duration of survival as a parameter of mortality rate assessment.
Statistical analysis
The Instat program (GraphPad Software Inc., San Diego, CA, USA) was used for statistical analysis. Hypotheses were tested by analysis of variance (ANOVA) and the χ2 test. Analyses of survival and the Cox regression model were used to correlate survival with metabolic syndrome and malnutrition. Statistical hypotheses were tested at the probability of significance, p < 0.05.
RESULTS
The BMI of the 407 patients was 20.6 ± 3.4 kg/m2 and the waist girth was 95 ± 13.2 cm. There were 81 (19.9%) patients who smoked cigarettes, and 144 (35.4%) had preserved diuresis. CVD was verified in 185 (45.5%) patients; 91 (22.3%) patients had diabetes mellitus and 72 (17.7%) had peripheral vascular disease. The group of patients treated in the HD program numbered 232 aged 59 ± 12.1 years, among whom were 150 (64.6%) males and 82 (35.4%) females. They had been on dialysis for 46 ± 54.4 months. Their BMI was 20.1 kg/m2 and waist girth 90 ± 12.2 cm. In this group of subjects, there were 55 (23.7%) who smoked cigarettes, 74 (32%) with diuresis, 94 (40.5%) patients had some manifestations of CVD, 21 (9%) had diabetes mellitus, and 28 (12.06%) had peripheral vascular disease. In the group treated by PD, there were 175 patients, 92 (52.6%) males and 83 (47.4%) females, of average age 71 ± 12.0 years; dialysis duration was 11 ± 17.5 months, BMI 21.2 ± 3.3 kg/m2, and waist girth 102 ± 9.8 cm. There were 26 (14.8%) smokers, whereas 70 (40%) individuals had preserved residual diuresis, CVD was verified in 91 (52%) patients; 70 (40%) had diabetes mellitus, and 44 (25%) had peripheral vascular disease. During our study, 53% of patients died. Among the HD-treated group, 37% died during the 4-year period, but among the PD group 65% died in the same period ().
TABLE 1. Demographic, anthropometric and clinical characteristics of the examined patients
The frequency of a lethal outcome was significantly higher in the group of PD-treated patients. Statistically significant differences in relation to metabolic changes were noted between the group of HD patients and the group of patients who chose PD treatment for kidney insufficiency, p < 0.001 (). Thus, patients with metabolic syndrome were dominant among the HD patients (51%), whereas 16% were undernourished. In the PD group, there were an almost equal number of patients with metabolic syndrome (38%) and malnutrition (37%).
TABLE 2. Mortality of examined patients in relation to metabolic changes
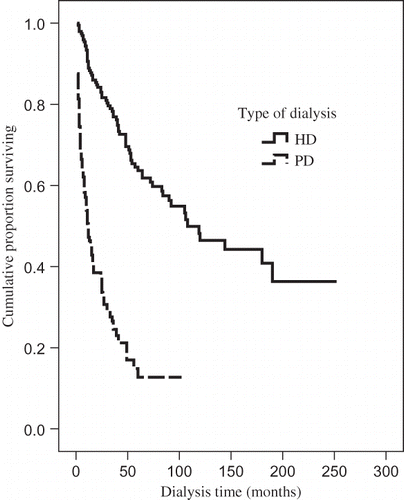
The median survival of HD patients was 108 months, 95% confidence interval (CI) 65–151, whereas that for PD patients was 12 months, 95% CI 8.7–15.3. Survival was significantly longer among the HD patients, p < 0.001 ().
FIGURE 1. Kaplan–Meier curve of survival in patients treated by HD and PD. Median of survival of HD patients was 108 months, confidence interval (95% CI 65–151). Median of survival of PD patients was 12 months, confidence interval (95% CI 8.7–15.3); p < 0.001.
Using the Cox regression model, it was determined that metabolic syndrome was a significant predictor of lethal outcome, that is, it was linked to shorter survival among chronic HD-treated patients (β = 0.560; p = 0.045). The presence of CVD approached statistical significance (β = 0.425; p = 0.072) in the sense of shorter survival. BMI (β = 0.088; p = 0.002) and waist girth (β = 0.023; p = 0.031) were significant predictors of lethal outcome among PD-treated patients, higher values being linked to shorter survival. Malnutrition (β = 0.454; p = 0.066) and inorganic phosphorus (β = −0.318; p = 0.095) were close to statistical significance in relation to shorter survival of PD patients ().
TABLE 3. Cox regression analysis of the expected parameters of survival in the examined patients
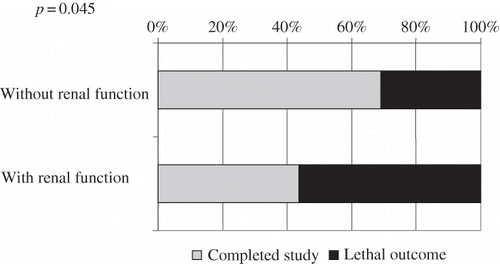
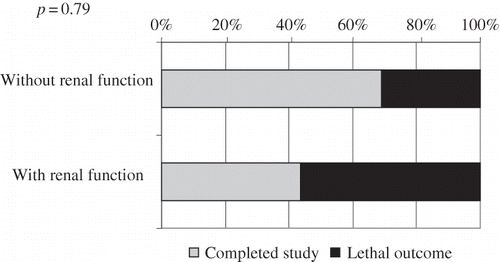
Among the PD patients, 40% of those who had preserved diuresis died, whereas 56% of those without residual function died. The difference was statistically significant (p = 0.045; ). On the contrary, there was no difference in mortality between HD patients with preserved diuresis and anuric HD patients (p = 0.79; ).
DISCUSSION
In the last three decades, the number of patients suffering from terminal kidney insufficiency has increased worldwide, and dialysis therapy remains the dominant form of treatment. Up to now there has been no consensus about the form of dialysis treatment that would offer patients the best chance of long-term survival.Citation2 The number of old persons, diabetics, and women is increasing as a subpopulation with the problem of creating a vascular approach. This imposes PD because of lack of transplantation as a priority model of depuration.Citation7 In France, 4 out of 10 patients who commence dialysis are older than 75 years, and every fifth dialysis starts as PD.Citation8 Such findings are diametrically opposite to data from the Canada–USA (CANUSA) study, which found that the average age of PD patients in the United States and Canada is 44.4 ± 13.1 years.Citation9 The average age of our PD patients was 71 ± 12.0 years, whereas that of the HD patients was 59 ± 12.1 years. Diabetes mellitus, as an etiological category of terminal kidney insufficiency, was present in 40% of the PD patients and 32% of the HD patients. In addition, the percentage of female PD patients was higher (46%) than for HD patients (35% female). In our study, these parameters were of less importance for the decision about which dialysis procedure to be used than the parameters concerning mortality risks factors, particularly regarding PD patients.
The mortality of dialysis patients can be extremely high.Citation10 In the United States, about one-third of such patients die during the first 5 years of treatment, which is a higher mortality rate than that for many malignant diseases.Citation2 In our 4-year study, slightly more than half of the patients died, but the mortality rate of HD patients was 37%, whereas among the PD patients two-thirds had a lethal outcome. Among our PD patients, the frequency of diabetes mellitus (40%) was greater than the incidence of diabetics in the European dialysis population older than 60 years,Citation8,Citation11 which ranged from 18 to 36%. Moreover, the frequency of CVD in this group (52%) was also markedly higher than the incidence of CVD in the European dialysis population (25–37%), which is another contributing factor to the high mortality of PD patients in our study.
Duration of dialysis is one mortality risk factor for dialysis patients.Citation11,Citation12 In relation to the duration of dialysis, the cumulative survival was significantly higher among HD patients than the PD patients in our study. Thus, average dialysis duration for our HD patients was 46 ± 54.4 months, whereas among PD patients it was 11 ± 17.5 months. These findings are not in accordance with those of other authors, which can be explained by the larger number of mortality risk factors among the group of PD-treated patients. Many studies have concluded that, compared to that for PD patients, the relative mortality risk for HD patients decreases with time in favor of the HD patients, which conditions the survival analysis of such patients with the duration of the observation study. Therefore, the results for chronic dialysis patients differ from those for new patients. Also, various studies have shown significant differences of survival between PD and HD patients, when diverse subpopulations, defined according to age or existence of diabetes mellitus, are compared, and patients may also change their way of dialysis treatment from one to another method.Citation13–15 Another source of confusion could be the issue of methodological processing of data.Citation16 Thus, differently formulated assumption strategies of survival of these patients may have contributed significantly to differences in the conclusions in those studies. We are aware of certain limitations of our investigation. First of all, it did not take into consideration all known risk factors that might have had an impact on mortality of our patients, such as differences in the type of HD and PD treatment, adequacy of dialysis, the unit dose of dialysis, the way of regulating of anemic status and metabolism of calcium and phosphorus, and expected complications that are common for such treatment. In addition, data about variability of body weight and changes in laboratory parameters during the period of study are missing. An important issue is also the question of whether HD was commenced through a catheter or not, because that increases the risk of septicemia exponentially and significantly influences the mortality of dialysis patients. All these factors of limitation made it harder to homogenize our group of patients into investigative subcategories.
Although the relation of modalities of dialysis procedure and the outcome of the treatment with the level of nutrition was not of interest in cohort studies, a positive association of mortality rate and BMI of 26 kg/m2 or more was observed in some investigations.Citation17–19 Williams et al.Citation20 found no proof that PD patients are more obese than those on HD, and – what is more important – that obesity of dialysis patients is not correlated with increased risk of getting CVD for the general population. On the contrary, low BMI and other parameters of underfeeding were strong independent indicators of mortality for dialysis patients. However, other studiesCitation21 indicate that obese PD patients have significantly poorer survival. It is interesting that obesity is associated with increased mortality rate – when we take obesity as a variable in multivariate analysis – in relation to normally fed or underfed patients. This indicates that the diverse findings could be explained by differences in the applied statistical models, which implies that the relation between BMI and mortality rate in the general and dialysis populations could be linear. The contradiction indicating that increased BMI in PD patients is a significant mortality factor in contrast to HD patients, for whom it is claimed to be a predictive parameter of survival, could be due to less efficient diffusion and osmosis in obese PD patients. Our results showed a significant difference between the group of patients treated by PD and those on the chronic HD program, in relation to the presence of metabolic syndrome or undernourishment. Metabolic syndrome was a significantly more frequent metabolic disorder among persons on HD treatment than malnutrition. When we analyzed the survival of patients on the PD and HD programs by Cox regression model in relation to the known risk factors, we found that metabolic syndrome of HD patients was a significant mortality risk factor, whereas among PD patients waist girth and BMI were predictive parameters of mortality. Undernourishment approached statistical significance among these patients which suggests a possible influence on mortality of patients treated by PD, which is identical with findings of the mentioned study.
Several recent reportsCitation9,Citation22–24 verified that residual renal function is a strong predictor of survival of PD patients, that is, that residual diuresis of 0.5–1 mL/min decreases mortality of these patients by 12%, or that every 250 mL of urine from PD patients increases survival by an additional 36%. It is important to point out that all those studies concluded that residual diuresis is an important benefit for dialysis patients, particularly for patients treated by PD, because the relative mortality risk – as a result of preserved diuresis – decreases on commencement of dialysis. There are many hypotheses about the connection between dialysis modalities and mortality incidence of obese patients with end-stage renal disease. The reason for increased mortality of patients treated by PD most probably could be found in nonadequate dialysis, particularly because of phased loss of residual renal function.Citation17 Residual renal function was a significant factor of survival of our PD patients, which confirms most studies that verified a positive correlation between residual diuresis and survival of PD patients.
There are also a few studies about the impact of residual diuresis on the survival of HD patients that reported a lower mortality risk among patients with preserved residual diuresis.Citation11,Citation25 Our results, however, did not indicate any significant impact of residual renal function on the survival of patients treated by HD.
In conclusion, more than half of our tested patients died during the 4-year period of the study. The cumulative survival rate was longer for HD patients. Metabolic syndrome was a predictive parameter of mortality, and malnutrition was a significant metabolic disorder among the HD patients. The predictors of mortality outcome among the PD patients were waist girth and BMI. Residual renal function was an important factor in reducing mortality in patients on PD.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Chung SH, Heimbürger O, Lindholm B, Lee HB. Peritoneal dialysis patient survival: A comparison between a Swedish and a Korean centre. Nephrol Dial Transplant. 2005;20:1207–1213.
- Khawar O, Kalantar-Zadeh K, Lo WK, Johnson D, Mehrotra R. Is the declining use of long-term peritoneal dialysis justified by outcome data? Clin J Am Soc Nephrol. 2007;2:1317–1328.
- James HG, Hans L, Melvin M. Initial survival advantage of peritoneal dialysis relative to hemodialysis. Nephrol Dial Transplant. 2002;17:112–117.
- Segall L, Covic A. Cardiovascular disease in hemodialysis and peritoneal dialysis: Arguments pro hemodialysis. Nephrol Dial Transplant. 2007;22:59–63.
- Gokal R. Peritoneal dialysis in the 21st century: An analysis of current problems and future developments. J Am Soc Nephrol. 2002;13(Suppl):S104–S115.
- Stolic R, Trajkovic G, Peric V, Frequency and characteristics of metabolic disorders in patients on hemodialysis. Vojnosanit Pregl. 2008;65:205–209 (Serbian).
- Winkelmayer WC, Glynn RJ, Mittleman MA, Levin R, Pliskin JS, Avorn J. Comparing mortality of elderly patients on hemodialysis versus peritoneal dialysis. A propensity score approach. J Am Soc Nephrol. 2002;13:2353–2362.
- Couchoud C, Moranne O, Frimat L, Labeeuw M, Allot V, Stengel B. Associations between comorbidities, treatment choice and outcome in the elderly with end-stage renal disease. Nephrol Dial Transplant. 2007;22:3246–3254.
- Kim SG, Kim NH. The effect of residual renal function at the initiation of dialysis on patient survival. Korean J Intern Med. 2009;24:55–62.
- Portoles J, del Peso G, Fernandez-Reyes MJ, Bajo MA, Lopez-Sanchez P, The GCDP. Previous comorbidity and lack of patient free choice of technique predict early mortality in peritoneal dialysis. Perit Dial Int. 2009;29:150–157.
- Termorshuizen F, Korevaar JC, Dekker FW, Hemodialysis and peritoneal dialysis: Comparison of adjusted mortality rates according to the duration of dialysis: Analysis of the Netherlands Cooperative Study on the Adequacy of Dialysis 2. J Am Soc Nephrol. 2003;14:2851–2860.
- Sigrist M, Bungay P, Taal MW, McIntyre CW. Vascular calcification and cardiovascular function in chronic kidney disease. Nephrol Dial Transplant. 2006;21:707–714.
- Bloembergen WE, Port FK, Mauger EA, Wolfe RA. A comparison of cause of death between patients treated with hemodialysis and peritoneal dialysis. J Am Soc Nephrol. 1995;6:184–191.
- Van Biesen W, Verbeke F, Vanholder R. Cardiovascular disease in hemodialysis and peritoneal dialysis: Arguments pro peritoneal dialysis. Nephrol Dial Transplant. 2007;22:53–68.
- Heaf JG, Lokkegaard H, Madsen M. Initial survival advantage of peritoneal dialysis relative to hemodialysis. Nephrol Dial Transplant. 2002;17:112–117.
- Vonesh EF, Moran J. Mortality in end-stage renal disease: A reassessment of differences between patients treated with hemodialysis and peritoneal dialysis. J Am Soc Nephrol. 1999;10:354–365.
- Inrig JK, Sun JL, Yang Q, Briley LP, Szczech LA. Mortality by dialysis modality among patients who have end-stage renal disease and are awaiting renal transplantation. Clin J Am Soc Nephrol. 2006;1:774–779.
- Garcia-Lopes MG, Agliussi RG, Avesani CM, Nutritional status and body composition after 6 months of patients switching from continuous ambulatorial peritoneal dialysis to automated peritoneal dialysis. Braz J Med Biol Res. 2008;41:1116–1122.
- Kalantar-Zadeh K, Kopple JD, Regidor DL, A1C and survival in maintenance hemodialysis patients. Diabetes Care. 2007;5:1049–1055.
- Williams JD, Woods HF. Insulin resistance, the metabolic syndrome and renal failure – is there a special problem for patients treated with peritoneal dialysis? European Endocrine Review. 2006;29–34.
- McDonald SP, Collins JF, Johnson DW. Obesity is associated with worse peritoneal dialysis outcomes in the Australia and New Zealand patient populations. J Am Soc Nephrol. 2003;14:2894–2901.
- Haag-Weber M. The impact of residual diuresis on survival. Nephrol Dial Transplant. 2008;23:2123–2126.
- Bargman JM, Golper TA. The importance of residual renal function for patients on dialysis. Nephrol Dial Transplant. 2005;20:671–673.
- Moist LM, Port FK, Orzol SM, Predictors of loss of residual renal function among new dialysis patients. J Am Soc Nephrol. 2000;11:556–564.
- Shemin D, Bostom, AG, Laliberty P, Dworkin LD. Residual renal function and mortality risk in hemodialysis patients. J Am Kidney Dis. 2001;38:85–90.