Abstract
We present an 18-year-old patient with Henoch–Schönlein purpura (HSP) who had multiple episodes of severe acute renal failure, including one episode for which he required hemodialysis for 2 months and a second episode for which dialysis was considered before his spontaneous recovery of renal function. Multiple treatment options, including steroids, mycophenolate mofetil, cyclophosphamide, and plasmapheresis, were tried but we could not confidently point to the utility of any of these measures. We highlight the unusual severity and lability of our patient's clinical course and how such a course makes the evaluation of treatment effectiveness extraordinarily difficult.
INTRODUCTION
Henoch–Schönlein purpura (HSP) is a systemic leukocytoclastic vasculitis mediated by IgA immune complex deposition, which affects small vessels in the skin, joints, gastrointestinal tract, and glomeruli. The disease is characterized by nonthrombocytopenic purpura concentrated in the lower extremities, arthritis/arthralgias, abdominal pain, and hematuria/renal disease. It is the most common vasculitis in childhood (annual incidence of 10–20 cases per 100,000 children)Citation1 and, less commonly, also occurs in adults. Adults generally suffer from more severe disease with a higher risk of severe renal involvement.Citation2
Here we report an 18-year-old male who developed severe HSP nephritis (HSPN). His course is notable and unusual for its dramatic renal relapses and remissions, including a 2-month period on dialysis. The implications of this undulating clinical course for both prognosis and therapy are discussed.
CASE REPORT
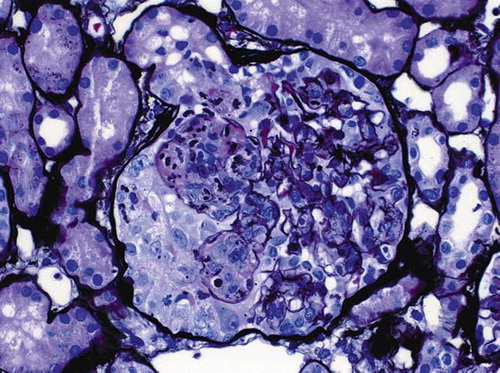
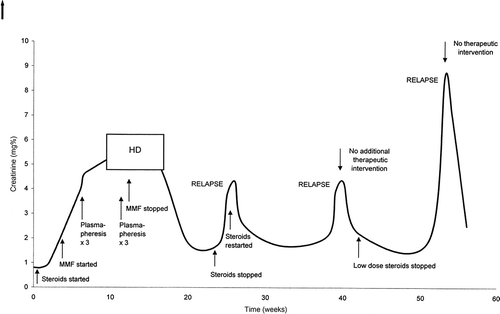
The patient is a Hispanic male, single, college student. He has no history of alcohol or recreational drug use. At the age of 18, he developed a purpuric rash, especially over the ankles and knees, joint swelling primarily in the lower extremities, some abdominal pain, and hematochezia. He was observed in the ER where a diagnosis of HSP was made and he was started on steroids. Two weeks later, he was admitted to the hospital due to persistent pain. His renal function was normal, with a serum creatinine of 0.8 mg%, but he had microscopic hematuria, trace proteinuria, and hypertension. A biopsy of his rash did not show vasculitis. Antinuclear antibodies (ANA), anti-DNA, anti-RNP, hepatitis studies, antineutrophil cytoplasmic antibodies (ANCA) studies, and complement levels were all negative or normal. Urine protein/creatinine ratio was approximately 0.4. He was administered intravenous (IV) steroids with improvement of his symptoms and was discharged on prednisone 20 mg bid. He was readmitted several days later with severe abdominal pain. During that hospitalization, he was noted to have nephrotic range proteinuria, severe hypertension, and had a renal biopsy that showed focal necrotizing and proliferative glomerulonephritis with occasional crescents (). His serum creatinine was 1.0 mg%. Mycophenolate mofetil was added. Two weeks later his serum creatinine was 4.7 mg%. Urinalysis showed 3+ protein and 3+ blood. He was pulsed with solumedrol and plasmapheresis was initiated. After the third plasmapheresis treatment, he developed massive gastrointestinal bleeding. Colonoscopy showed diffuse colitis. He was switched to IV solumedrol at a dose of 30 mg every 6 h and this was eventually tapered and switched back to oral steroids. Because of worsening renal function, he was started on hemodialysis. Three further plasmapheresis treatments were also performed. He had a repeat kidney biopsy, which showed IgA nephropathy with extensive crescent formation, with very little interstitial fibrosis. He was discharged on methylprednisolone 40 mg twice a day and mycophenolate mofetil 500 mg twice a day. Mycophenolate mofetil was discontinued about 10 days later and the steroids were progressively reduced over the next 2 months and then discontinued. His renal function slowly improved and hemodialysis was discontinued after 2 months of treatment. His serum creatinine came down to 1.9 mg% and his proteinuria significantly improved. His hypertension also became easier to manage. Unfortunately, 4 days after discontinuation of steroids, he developed gross hematuria, diarrhea, and abdominal pain. His serum creatinine rose to 3.8 mg%. His urinalysis showed 3+ protein and 3+ blood. An abdominal CT showed some colitis, but colonoscopy was normal. Serologic evaluation, including ANA, ANCAs, and complements, were again either negative or within normal limits. A repeat renal biopsy showed more scarring and fibrosis. He was restarted on high-dose steroids and his renal function began to improve from a peak value of 4.3 to 2.6 mg%. He was discharged on prednisone 120 mg every other day. His steroids were again gradually tapered and his serum creatinine decreased to 1.7 mg%. Two months later, he had a flu-like illness, a few days after which he developed gross hematuria, with worsening proteinuria and a creatinine increase to 4.3 mg/dL. However, with no specific therapy, his hematuria resolved in a few days and his creatinine returned to the 2.2-mg% range. Three months later, his creatinine level was 1.9 mg%, but he then had another renal exacerbation and his creatinine rose to 8.6 mg%. It was elected not to pursue aggressive treatment and to start the patient on hemodialysis. However, within 5 days his renal function once again began to improve and hemodialysis was not initiated. His serum creatinine went back to the mid-2’s (see for an overview of the clinical history).
DISCUSSION
HSP is a relatively rare disorder that has a highly variable clinical course and outcome. In a study of 250 adults with HSP, followed up for a median of 14.8 years, 11% reached end-stage renal disease (ESRD) and an additional 27% had moderate or severe renal insufficiency.Citation3
Various authors have identified differing prognostic indicators further complicating treatment evaluation. Shrestha et al.Citation4 evaluated 37 adults with HSPN. A total of 27% progressed to ESRD. The risk factors for ESRD were proteinuria ≥1 g/day during follow-up, hypertension, renal impairment at presentation, age <30 years, and male sex. Crescents and interstitial fibrosis on renal biopsy also predicted ESRD. However, 26% of their patients with crescents retained normal renal function. Cytotoxics were used in 32% of patients and had no clear effect on outcome. Pillebout et al.Citation3 followed 250 adults with HSP for a median period of 14.8 years. Multivariate analysis demonstrated that renal function impairment and proteinuria level at presentation were risk factors. The presence of crescents on renal biopsy was not a risk factor, but indicators of chronicity and the presence of fibrinoid necrosis were risk factors. They could not demonstrate a positive effect of treatment with immunosuppressive agents, but commented that the retrospective nature of their study precluded adequate treatment evaluation. Coppo et al.Citation5 reviewed 219 patients with HSP (83 children and 136 adults) followed up for a median of 4.5 years. They found that no data detected at diagnosis, including renal function impairment, proteinuria, hypertension, and crescentic nephritis (involving >50% of glomeruli in only 2.6%), were significantly related to functional decline with multivariate analysis. Increasing mean proteinuria levels during follow-up were associated with progression. Soylemezoglu et al.Citation6 in a study of 443 children with HSPN were unable to show any association between initial symptoms and histology with outcome. Finally, Rauta et al.,Citation7 in an evaluation of 38 Finnish patients with HSPN noted no histopathological findings that were associated with poor outcome. The only factor statistically significantly related to the progression of HSPN in their patients was a level of proteinuria greater than 1.0 g/24 h.
Treatment of HSP, particularly in adults, is far from clear-cut, with no protocol that has been agreed upon. A variety of quite different therapies in case reports or small series have been reported to be effective in the treatment of HSP. These therapies include steroids and azathioprine,Citation8 high-dose IV immunoglobulins,Citation9 tonsillectomy,Citation10 mycophenolate mofetil,Citation11 cyclophosphamide, plasmapheresis,Citation12 and thalidomide.Citation13 However, there have been no substantial randomized clinical trials (RCTs) of these agents in adults. In children, there have been seven steroid trials, involving nearly 650 children, evaluating prednisone therapy at presentation of HSP to prevent nephropathy and the results are conflicting.Citation1 Also in children, a trial of cyclophosphamide and supportive therapy or supportive therapy alone in 56 children with biopsy-proven HSPN followed for 6.9 years found no difference in outcome between the two groups.Citation14 Renal transplantation is another treatment option with suggested good long-term survival. However, there is a reported 42% recurrence rate, with loss of the graft in half of those patients.Citation15
Our patient's clinical course highlights the problems associated with evaluating and treating HSP. He has had an extraordinarily labile course with multiple exacerbations and remissions. He had a prolonged period on dialysis and then was able to discontinue dialysis. Remission after prolonged dialysis is rare, but its occurrence complicates our ability to make a confident prognosis. Torregrosa de Juan et al.Citation16 reported a patient who had two episodes of acute renal failure separated by approximately 1 year. Both episodes spontaneously reversed after 6 and 4 months on hemodialysis, respectively. Rech et al.Citation17 reported a patient, treated with steroids, cyclophosphamide, and plasma exchange, who recovered renal function after 55 days on hemodialysis.
Our patient's course also highlights the difficulty in evaluating therapy in HSP. There were times when we noted a temporal relationship between steroid therapy and steroid withdrawal and disease remission/exacerbation. Conversely, we also saw a similar pattern of remission/exacerbation when no treatment was administered. This treatment conundrum is possibly compounded by the observation of Pillebout et al.Citation3 that the most frequent cause of death in their group was neoplasia and that the second cause of death was infection with, two-third of infectious deaths attributed to immunosuppressive treatment. Death from HSP evolution was less likely. None of the above means that treatment is ineffective, but clinicians will have to carefully weigh the risks versus the benefits of treatment based on individual patient characteristics and their best estimate of patient prognosis.
Acknowledgment
We thank Nephropath and Chris Larsen, MD, for processing and evaluating the renal biopsy specimens.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this paper.
REFERENCES
- Bogdanović R. Henoch-Schönlein purpura nephritis in children: Risk factors, prevention and treatment. Acta Paediatr. 2009;98:1882–1889.
- Uppal SS, Hussain MAS, Al-Raqum HA, Henoch-Schönlein's purpura in adults versus children/adolescents: A comparative study. Clin Exp Rheumatol. 2006;24 (Suppl. 41): S26–S30.
- Pillebout E, Thervet E, Hill G, Alberti C, Vanhille P, Nochy D. Henoch–Schönlein purpura in adults; outcome and prognostic factors. J Am Soc Nephrol. 2002;13:1271–1278.
- Shrestha S, Sumingan N, Tan J, Althous H, McWilliam L, Ballardie F. Henoch–Schönlein purpura with nephritis in adults: Adverse prognostic indicators in a UK population. Q J Med. 2006;99:253–265.
- Coppo R, Andrulli S, Amore A, Predictors of outcome in Henoch–Schönlein purpura in children and adults. Am J Kidney Dis. 2006;47:993–1003.
- Soylemezoglu O, Ozkaya O, Ozen S, Henoch–Schönlein nephritis: A nationwide study. Nephron Clin Prac. 2009;112:c199–c204.
- Rauta V, Tornroth T, Gronhagen-Riska C. Henoch–Schönlein nephritis in adults-clinical features and outcomes in Finnish patients. Clin Nephrol. 2002;58:1–8.
- Bergstein J, Leiser J, Andreoli SP. Response of crescentic Henoch–Schönlein purpura nephritis to corticosteroid and azathioprine therapy. Clin Nephrol. 1998; 49:9–14.
- Kusuda A, Migita K, Tsuboi M, Successful treatment of adult-onset Henoch–Schönlein purpura nephritis with high-dose immunoglobulins. Internal Med. 1999;38:376–379.
- Sugiyama H, Watanabe N, Onoda T, Successful treatment of progressive Henoch–Schönlein purpura nephritis with tonsillectomy and steroid pulse therapy. Internal Med. 2005;44:611–615.
- Dede F, Onec B, Ayli D, Gonul II, Onec K. Mycophenolate mofetil treatment of crescentic Henoch–Schönlein nephritis with IgA depositions. Scand J Urol Nephrol. 2008;42:178–180.
- Hattori M, Ito K, Kawaguchi H, Yoshioka T, Khono M. Plasmapheresis as the sole therapy for rapidly progressive Henoch–Schönlein purpura nephritis in children. Am J Kidney Dis. 1999;33:427–433.
- Choi SJ, Park SK, Uhm WS, A case of refractory Henoch–Schönlein purpura treated with thalidomide. Korean J Int Med. 2002;17:270–273.
- Tarshish P, Bernstein J, Edelmann CM. Henoch–Schönlein purpura nephritis: course of disease and efficacy of cyclophosphamide. Pediatr Nephrol. 2004;19:51–56.
- Moroni G, Gallelli B, Diana A, Renal transplantation in adults with Henoch–Schönlein purpura: Long-term outcome. Nephrol Dial Transplant. 2008;23:3010–3016.
- Torregrosa de Juan E, Hernández Jaras J, Sánchez Canell JJ, Recurrent reversible acute renal failure in a patients with hematuria and Schönlein–Henoch purpura. Nefrologia. 2008;6:649–651.
- Rech J, Fuchs F, Kallert S, Plasmapheresis therapy in an elderly patient with rapidly progressive Henoch–Schönlein purpura with disseminated organ involvement. Clin Rheumatol. 2007; 26:112–114.