Abstract
Background/aims: Cytokine gene polymorphisms have been implicated as potential genetic risk factors for cardiovascular diseases (CVDs). Atherosclerosis and left ventricular hypertrophy (LVH) are surrogate markers for CVDs in uremic patients. The aim of this study was to assess the role of cytokine gene polymorphisms in carotid intima–media thickness (CIMT) and left ventricular mass index (LVMI) progression in nondiabetic hemodialysis (HD) patients.
Methods: About 102 nondiabetic patients on maintenance HD were included in this study. Patients were followed up for 2 years. Genetic polymorphisms of TNF-alpha (−308 G/A, −238A/G) and IL-10 (−1082 A/G, −819 C/T, −592 A/C) were determined by polymerase chain reaction. Biochemical parameters and inflammatory markers and ambulatory blood pressure (BP) measurements were determined during the study period. CIMT and LVMI were also determined at baseline and after the first and second year. Results: Cardiovascular risk factors did not differ between TNF-alpha −308 high-/low-producer genotype groups. However, CIMT and LVMI progression were detected at higher levels in patients with high-producer genotypes (AA+AG) than in patients with the low-producer genotype (GG) during the study period. The TNF-alpha −308 G/A polymorphism was closely associated with C-reactive protein (CRP), a marker of systemic inflammation in the study population. Analysis also showed that the combination of high production of TNF-alpha and low production of IL-10 was associated with higher average IMT and LVMI progression and elevated average CRP levels compared with a combination of low production of TNF-alpha and high production of IL-10. Conclusion: Polymorphisms in inflammatory genes may represent an additional factor affecting inflammation and CVD progression in nondiabetic HD patients.
INTRODUCTION
High prevalence of cardiovascular disease (CVD) in uremic patients cannot be fully explained by traditional risk factors, and alternative mechanisms, specific to uremia or dialytic treatment, have been suggested. On the basis of experimental and clinical data, C-reactive protein (CRP), the prototype marker of inflammation, is strongly associated with CVD in end-stage renal disease (ESRD).Citation1 Atherosclerosis and left ventricular hypertrophy (LVH) are well-known risk factors for cardiovascular morbidity and mortality in CVD patients,Citation2,Citation3 and the major contribution of inflammatory mechanisms to atherosclerosis and LVH in uremic patients has recently been emphasized.Citation4,Citation5
The inflammatory cascade which ultimately leads to elevated levels of CRP is triggered by the activation of circulating monocytes and of macrophages at the site of tissue injury. Autocrine and paracrine activation of monocytes plays a key role in this process. Cytokines are regulatory mediators of monocyte and macrophage activation. Two cytokines, tumor necrosis factor alpha (TNF-alpha) and interleukin-10 (IL-10), have complex and predominantly opposing roles in inflammation.Citation6,Citation7 TNF-alpha is one of the most potent autocrine activators of monocytes and also upregulates the synthesis of cytokines. IL-10, on the other hand, inhibits macrophage function, including cytotoxic activity and cytokine synthesis. An autoregulatory loop appears to exist in which TNF-alpha stimulates IL-10 production, which in turn reduces TNF-alpha synthesis.Citation8
The pattern and magnitude of cytokine release are considered critical determinants of severity and duration of response. Interindividual differences in the regulation of IL-10 and TNF-alpha production may be critical in a variety of healthy and abnormal inflammatory responses. A number of polymorphisms located close to or within the IL-10 and TNF-alpha genes are potentially associated with transcription levels.Citation9,Citation10 The best documented of these polymorphisms are the IL-10 promoter polymorphisms −1082G/A, −819C/T, and −592C/A and the TNF-alpha promoter polymorphisms −238 G/A and −308G/A. Polymorphisms at the position −308 G→A and −238 G→A in the promoter region of the TNF-alpha gene are associated with higher rates of transcription.Citation9 The best-documented IL-10 gene promoter polymorphisms, also associated with higher transcription levels, are −1082 A→G, −819 T→C, and −592 A→C.Citation10 Recently, cytokine gene mutations were implicated in the pathogenesis of CVD. Findings from clinical studies showed that the TNF-alpha gene polymorphism is associated with coronary artery disease and myocardial infarction.Citation11–13 The IL-10 −1082 A allele, associated with low production of IL-10, is also predictive of higher cardiovascular morbidity in dialysis patients.Citation14
We aimed to evaluate the interrelationships between inflammatory and/or anti-inflammatory cytokine gene polymorphisms and CRP, changes in left ventricular mass index (LVMI), and changes in carotid intima–media thickness (CIMT) in nondiabetic hemodialysis (HD) patients.
METHODS
Study population and study design
The study enrolled all dialysis patients (n = 102) who fulfilled the inclusion criteria in our dialysis unit. Exclusion criteria for the trial were as follows: diagnosis of chronic infectious disease, coronary artery disease, myocardial infarction, or cerebrovascular accident in the past 12 months; evidence for severe hepatic disease; use of immunosuppressant or nonsteroidal anti-inflammatory drugs (NSAIDs); congestive heart failure; the presence of a malignant disease or noncompliance of the subjects; valvular heart disease; other vascular diseases; and diabetic nephropathy (diabetes mellitus is a major independent promoter of atherosclerosis). They were followed up for 2 years.
All patients' treatment was regular bicarbonate standard dialysis that lasted 4–5 hours, three times per week (mean ± SD duration, 42.8 ± 8.1 months), with dialyzer surface area ranging from 1.3 to 1.8 mCitation2. All of the patients were treated using synthetic membranes (mainly polyamide and polysulfone). Dialysis period and dialyzer type were prescribed individually based on a urea-kinetic model to maintain Kt/V >1.2. Dry body weight was evaluated on a clinical basis with the aid of chest radiographs performed every 3 months to assess cardiothoracic index. Vascular access was via arteriovenous fistula in 98 patients. A permanent intravenous catheter was used in the others.
All patients were administered intermittent subcutaneous individually adjusted doses of recombinant human erythropoietin to keep hemoglobin levels between 10 and 12 g/dL. Most patients were treated with oral or intravenous calcitriol supplements and calcium carbonate or acetate and sevelamer (n = 4) tablets, if required. Patients with hypertension (n = 16) (24 h ambulatory blood pressure (BP), systolic and diastolic BP > 135/85 mmHg) were also treated with anti-hypertensive medication.
The study was carried out in accordance with the Declaration of Helsinki (1989) and informed consent was obtained for all patients. Study protocol has been approved by Hacettepe University Ethic Committee on Human Research.
The duration of the study was 24 months. Follow-up visits were scheduled at 3 monthly intervals. Follow-up visits included clinical assessment and measurement of 24 h ambulatory BP and routine laboratory tests. All patients underwent a 2D guided M-mode echocardiography and B-mode carotid ultrasonography at baseline and 6 monthly intervals of follow-up. All patients in the study were evaluated for serious adverse events that were defined as death and any abnormal laboratory value associated with signs or symptoms or necessitates treatment during 24 months. All alterations in medications were recorded.
Blood pressure measurements
Ambulatory BP was measured over a 24 h period by the oscillometric method using an automatic noninvasive recorder (Spacelab Inc., Redmond, WA, USA) on the following day after dialysis. The monitor was programmed to measure BP at 15 min intervals between 8:00 a.m. and 10:00 p.m. and at 30 min intervals between 10:00 p.m. and 8:00 a.m. During measurement, patients performed their usual regular daily activities. Measurements were only included if more than 85% of the readings were successful. Mean 24 h systolic BP, diastolic BP, and mean arterial pressure (MAP) were recorded at baseline and following visits in all patients.
Echocardiography
A Vingmed CFM 750 ultrasonographic device system (GE Vingmed Sound, Horten, Norway) with a duplex mechanical annular array probe was used. Signals from the last 10 s are stored in an internal replay memory where they can be recalled or transferred to external computers. The patients were examined in the lateral recumbent position after 30 min of rest. Dimensions were measured by M-mode echocardiography. The same examiner performed recordings at baseline and on every follow-up at 6-month intervals. In our laboratory, the intra-observer variability is below 10% for LVMI (4.6 ± 1%). Recordings from each patient were analyzed at the same time to ensure consistent measurement technique using a devoted analysis program (Echopac, GE Vingmed Sound). Dimensions and chamber function assessed by ejection fraction (EF) of the left ventricle were measured using the recommendations of the American Society of EchocardiographyCitation15. The two-dimensional left ventricular endocardium was traced at end diastole and peak systole in cine loops of apical four-chamber and two-chamber views, and the correct positions of the tracings were controlled by running the cine loops.
Left ventricular mass (LVM) was calculatedCitation7 as
where LVIDd is the left ventricular diastolic dimension; PWTd the diastolic dimension of posterior wall; and SWTd the diastolic dimension of interventricular septum, in centimeters. LVM was normalized for body surface area for calculation of LVMI.
LV hypertrophy was identified by validated gender-specific partition values of LV mass index, ≥116 g/m2 in men and 96 g/m2 in women.Citation15
Carotid ultrasonography
The CIMT was measured by one trained radiologist without knowledge of the clinical data. CIMT, defined as the distance between the media–adventitia interface and the lumen–intima interface, was measured using a duplex ultrasound system with a 7.5 MHz scanning frequency in the B-mode, pulsed Doppler mode, and color mode (Siemens Elegra Ultrasonography Systems, Erlangen, Germany). The far-wall IMT was measured at three different locations on both sides: one measurement from internal carotid artery (ICA), one measurement from bifurcation enlargement (BIF), and three measurements from common carotid artery (CCA) (proximal, middle, and distal from the bifurcation) as reported previously.Citation16,Citation17 The mean CIMT was defined as the mean of right and left ICA, BIF, and the three highest CCA measurements. The reproducibility of the CIMT measurements was examined by conducting another scan 1 week later on eight subjects. In our laboratory, the intra-observer variability is below 10% for CIMT (4.5 ± 3.1%) demonstrating good reproducibility of repeated measurements. The common, internal, and external carotid arteries were also scanned longitudinally and transversely to assess occurrence of plaques. We defined the presence of carotid plaque as intima–media thickening that exceeded more than 1.0 mm. CIMT was always performed at plaque-free regions.
Laboratory measurements
Biochemical parameters (creatinine, blood urea nitrogen, glucose, sodium, potassium, calcium, phosphate, albumin, hemoglobin), total cholesterol, triglyceride, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and inflammatory markers (CRP, erythrocyte sedimentation rate, white cell count) were determined at the initiation and at 3-month intervals during the 2-year study period. CRP was detected during the first week of the month regardless of whether the patient had intercurrent complications. Annual mean values of biochemical parameters were calculated for each patient. Biochemical parameters were measured by means of a computerized auto-analyzer (Hitachi 717; Boehringer, Mannheim, Germany). Total cholesterol (enzymatic cholesterol oxidase peroxidase (CHOP) – the peroxidase-antiperoxidase (PAP) method, Boehringer-Mannheim, Mannheim, Germany) and triglycerides (TG) were quantified by commercial colorimetric assay method (peridochrom alpha glycerol phosphate oxidase GPO-PAP method; Boehringer-Mannheim). HDL-C was quantified by the phosphotungstic acid precipitation method. LDL-C was calculated by Friedewald formula (LDL-C = CHO – TG/5 – HDL-C).Citation18 Serum i-PTH concentrations were determined by dual antibody radioimmunoassay (Nichols Institute, San Juan Capistrano, CA, USA). Erythrocyte sedimentation rate was determined by the Sedi-system (Becton Dickinson, Paris, France) and CRP was detected by rate nephelometry (IMAGE, Beckman, Brea, CA, USA).
Genotypes
Venous blood (10 mL) was collected into tubes containing 50-mmol disodium EDTA and genomic DNA was isolated with a DNA extraction kit (Protrans, Quest Biomedical, Solihull, UK). All donors were genotyped for IL-10 and TNF-alpha using a PCR sequence-specific assay (Cyclerplate System Cytokine, Protrans, Ketsch, Germany). The assay consists of ready-to-use primer mixes prepipetted into thin-walled wells and includes eight IL-10-specific primers and four TNF-alpha-specific primers. Thermocycling conditions were as follows: initial denaturation at 94°C for 2 min, 10 cycles of denaturation at 94°C for 10 s with a single step of annealing and extension at 65°C for 1 min, followed by 20 cycles of denaturation at 94°C for 10 s, annealing at 61°C for 50 s, and extension at 72°C for 30 s. PCR products were analyzed by 1% agarose gel electrophoresis and visualized with ethidium bromide. The expected sizes of 20 different specific amplification products for each primer pair were evaluated in accordance with the instructions in the kit. Genotypes corresponding to each of the five polymorphisms being investigated (IL-10 promoter –1082 G→A, –819 C→T and –592 C→A, and TNF-alpha −308 A→G and –238 G→A) were determined.
Statistical analysis
The data are presented as means ± standard deviation. All statistical tests were two sided. Genotypes of single nucleotide polymorphisms (SNPs) were classified into two categories by three ways, including the major allele's homozygotic model, minor allele's homozygotic model, and major and minor alleles' heterozygotic model. The Mann–Whitney or Kruskal–Wallis test was applied to compare the average mean CIMT and LVMI progression for subjects with and without the SNP genotype or the SNP combination and ?2 or Fisher exact test statistics to evaluate differences in proportion. Genotypes of SNPs were also compared using analysis of covariance with baseline as the covariate for the change in echocardiography and ultrasonography findings. The data of age, sex, BMI, lipid profiles, hypertension, and smoking status were controlled for as covariates.
We performed multiple comparisons for five different polymorphisms and three different outcomes. A conservative approach would be to pull the level of statistical significance down to 0.0033 [0.05/(5 × 3)] to keep the overall type I error of the study within 5% for the 15 comparisons (if all the comparisons were independent). The analyses were also performed on the nine models of SNP combinations; correction for multiple testing is required. Bonferroni's multiple comparison procedure was utilized for the correction and gave the corrected level of significance, 5.5 × 10−4 [0.05/(5C2 × 9)] in the SNP combination analyses. Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS for Windows Software Package; 15.0, SPSS Inc., Chicago, IL, USA).
RESULTS
Demographic and laboratory baseline characteristics of study population are presented in . All patients were followed up prospectively after the baseline assessments. Of 102 patients, 92 completed the study through the entire 2 years. Among the 10 patients who did not complete the study, 4 discontinued because of death, 2 discontinued because of transplantation, 3 discontinued because of failure of laboratory measurements, and 1 discontinued because of transfer to chronic ambulatory peritoneal dialysis treatment.
TABLE 1. Baseline characteristics of the study population (102 chronic HD patients)
Genotyping IL-10 and TNF-alpha promoter polymorphisms
The allele frequency and genotype distribution of IL-10 −1082 G/A, −819 C/T, and −592 C/A and TNF-alpha −308 A/G and −238 G/A are shown in . A set of Hardy–Weinberg analyses on the genotype frequencies revealed that the study group is representative of a population with random matching for the IL-10 −1082, IL-10 −819, and IL-10 −592 loci, and the TNF-alpha −308 and TNF-alpha −238 loci (all p-values > 0.1). We observed the frequencies of alleles and allele combinations and genotype distributions to be in good correlation with the results of previous studies with Caucasian populations.Citation10–14 A total of 34% of the patients were homozygous for the base G at position −1082 (−1082 GG), a high-producer genotype for the coding sequence of the IL-10 gene. Similarly, 33% of the patients carried the base A (−308 AA+AG) at position −308 upstream of the coding sequence of the TNF-alpha gene, a high-producer genotype for TNF-alpha gene. indicates the characteristics of these patients, comparing high-producer and low-producer genotypes. There were no differences in terms of age, sex, body mass index, duration of HD, habitual smoking, medications, 24 h ambulatory BP levels, interdialytic weight gain, Kt/V, hemoglobin levels, serum albumin, and serum total cholesterol, LDL-C, HDL-C, and triglyceride levels at the study entry and during follow-up (). Furthermore, there were no differences in the distribution of underlying diseases between the genotype groups.
TABLE 2. Genotype distribution of the study population
TABLE 3. Baseline demographic and laboratory parameters (mean ± SD) according to cytokine genotype in study population
TABLE 4. Average parameters (mean ± SD) according to cytokine genotype during study period
CIMT and genotypes
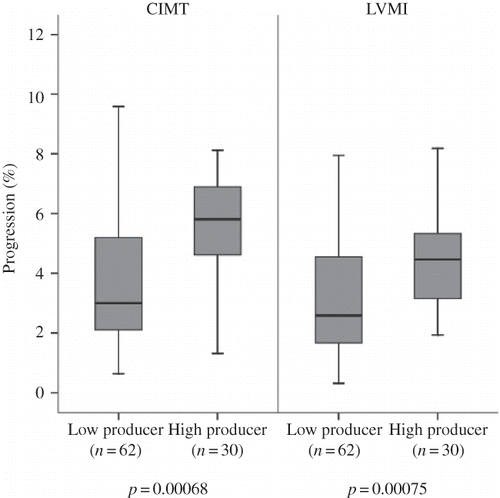
In the study population, 102 participants had CIMT scans at 0 months, 98 participants at 12 months, and 92 participants at 24 months. Measurements are presented in . After the 2-year follow-up, the mean CIMT of the study population had progressed by 8.2 ± 4.9% (p = 0.00024) suggesting an association with somewhat independent aspects of genotype effect. However, although baseline measurements did not differ between genotype groups, in the single SNP analyses, high-producer genotypes [TNF-alpha −308 (AA+AG)] were found to be associated with significantly higher annual average CIMT progression compared with low-producer (GG) genotypes (5.6 ± 2.5% vs. 3.5 ± 2.1%, respectively, p = 0.00068) (). Next, investigation of the association between annual average CIMT progression and the combinations of potential susceptible genes showed that the genotype combination of high producers of TNF-alpha [−308 (AA+AG)] and low producers of IL-10 (−1082 AA) was associated with higher average CIMT progression than the genotype combination of low producers of TNF-alpha (−308 GG) and high producers of IL-10 (−1082 GG) (6.2 ± 2.3% vs. 3.0 ± 1.8%, respectively, p = 3.3 × 10−4) (). The associations of CIMT measurements with genotype combinations are listed in . No significant genotype–subgroup interactions between mean CIMT and metabolic parameters such as hemoglobin level, erythrocyte sedimentation rate, and lipid levels were found for any subgroup considered. We could not find any significant association between the TNF-alpha −238 and the other IL-10 genotype groups and CIMT progression.
FIGURE 2. Annual LVMI and CIMT progression due to combination of TNF-α −308/IL-10 −1082 gene polymorphisms.
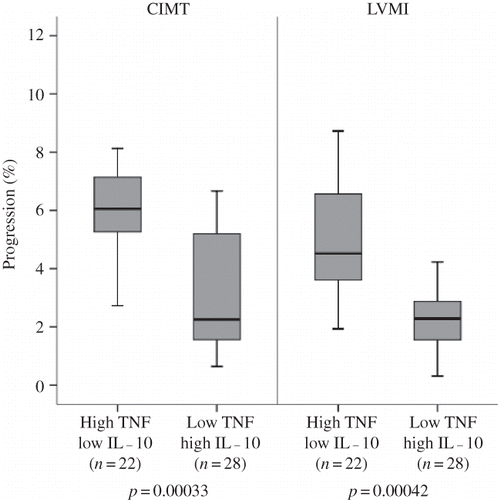
TABLE 5. LVMI and CIMT measurements in cytokine genotype groups
TABLE 6. LVMI and CIMT measurements in cytokine genotype combination groups
LVMI and genotypes
Correlations of genotype and echocardiographic data obtained from the 92 participants who completed the study are summarized in and . The study patients showed a significant progression in mean LVMI at the end of follow-up as compared with baseline measurements (6.6% ± 3.2%) (p = 0.00074) demonstrating the existence of risk factors for LVMI progression in the HD population that are independent of genotype. However, though this significant progression in mean LVMI was common to all participants, there were differences in the rate of progression between different genotype groups. A trend toward higher annual mean LVMI progression was observed in the high-producer group (TNF-alpha −308 genotype AA + AG) versus the low-producer group (GG) (4.4 ± 2.0 vs. 2.8 ± 1.5%, respectively, p = 7.5 × 10−4) (). Analyses of SNP combinations also revealed differences in LVMI progression. The combination genotypes giving rise to high production of TNF-alpha (−308 AA + AG) and low production of IL-10 (−1082 AA) were associated with greater LVMI progression than those giving rise to low production of TNF-alpha (−308 GG) and high production of IL-10 (−1082 GG) (4.6 ± 2.1 vs. 2.5 ± 1.5%, respectively, p = 4.2 × 10−4) (). Covariance analysis demonstrated that these genotype effects on LVMI progression were independent of age, sex, body mass index, duration of HD, habitual smoking, medications, 24 h ambulatory BP levels, interdialytic weight gain, hemoglobin, serum albumin, and serum lipid levels.
C-reactive protein and genotypes, CIMT, and LVMI
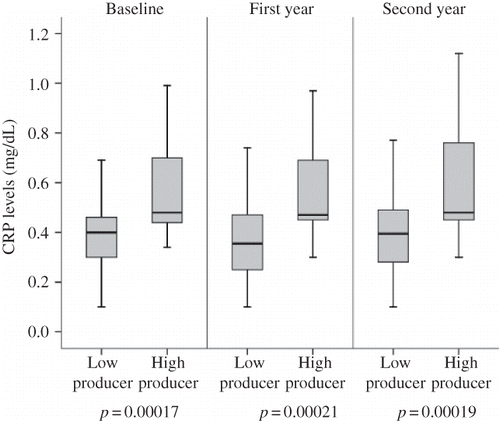
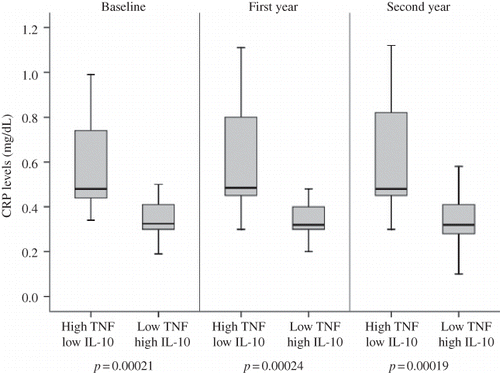
The TNF-alpha −308 G/A genotype was closely associated with CRP, a marker of systemic inflammation in the study population. CRP levels were higher in the TNF-alpha −308 AA + AG group compared with the −308 GG group at study entry (p = 0.00017). This association continued throughout the 2-year longitudinal observation. shows CRP levels of patients with high- and low-producer genotypes for TNF-alpha −308 during the study period. The TNF-alpha −308 high-producer genotype was associated with more frequent elevations of the acute protein compared with the TNF-alpha −308 low-producer genotype. This finding suggested that the TNF-alpha −308 high-producer genotype may trigger subclinical inflammation. The TNF-alpha −238 and IL-10 genotype groups did not show a significant association with CRP levels. In the combination genotype analyses, high producers of TNF-alpha (−308 AA + AG) and low producers of IL-10 (−1082 AA) had significantly higher average CRP levels compared with low producers of TNF-alpha (−308 GG) and high producers of IL-10 (−1082 GG) at the beginning of the study (p = 2.1 × 10−4). This difference also sustained during the study period ().
At baseline, CIMT and LVMI did not correlate with inflammatory markers. However, mean CRP was positively correlated with the rate of progression of CIMT and LVMI measured during the 2-year trial period (r = 0.25, p = 0.023 and r = 0.65, p = 0.01, respectively).
DISCUSSION
This study investigated the association between functional variants in the TNF-alpha and IL-10 genes, encoding cytokines involved in the inflammatory axis implicated in LVH and atherosclerosis progression in patients on HD. A combination of two polymorphisms was found to be associated with increased progression of LVMI and CIMT and increased serum CRP levels in patients on HD. These two polymorphisms were the TNF-alpha gene −308 A/G polymorphism, leading to high production of TNF-alpha, and the IL-10 gene −1082 polymorphism, leading to low production of IL-10. These effects seem unrelated to the other cardiovascular risk factors. The IL-10 −819C/T, −592 C/A, and TNF-alpha −238 A/G gene polymorphisms had no measurable influence on the progression of CIMT or LVMI.
It has been previously reported that CRP, the prototype marker of inflammation, is strongly associated with LVH and atherosclerosis in both ESRDCitation5,Citation19 and non-ESRD patients.Citation20–22 In the general population, absolute values usually stayed within the normal range; in contrast, dialysis patients showed chronically elevated CRP levels even in the absence of infectious complications. However, there were interindividual differences in the extent of inflammation that cannot be fully explained by uremia or HD. Studies have shown that the extent of chronic inflammation in dialysis patients is related to the individual's production of regulatory cytokines.Citation23 Individual genetic differences determining the amount of inflammatory or anti-inflammatory cytokines produced may explain the large interindividual differences in the inflammatory activation seen in these patients. Furthermore, due to inflammatory activation, genetic variation in cytokine levels may influence cardiovascular markers in HD patients.
The association between plasma levels of TNF-alpha and CRP supports the view that monocyte activation may lead to elevated plasma levels of CRP via intermediate steps in the inflammatory cascade.Citation24 The present study showed that the polymorphism at position −308 of the TNF-alpha gene promoter, which determines a ‘high-producer’ or ‘low-producer’ phenotype, is predictive of risk of atherosclerosis and LVH in HD patients. This finding is independent of many accompanying traditional and/or uremia-related cardiovascular risk factors. TNF-alpha is a potent immunomodulator and proinflammatory cytokine that has been implicated in the pathogenesis of inflammatory and infectious diseases.Citation25 As interindividual differences in TNF-alpha release have been described, a TNF-alpha G→A transition at position −308 may be an important functional polymorphismCitation9,Citation26 that affects both the prevalence of inflammation and its associated complications in ESRD. In the general population, the −308 G→A transition is associated with a state of high TNF-alpha production and susceptibility to several diseases.Citation9,Citation27 Over-expression of TNF-alpha has been also implicated in the pathogenesis of CVD. However, association between polymorphisms in the TNF-alpha gene and CVD in the general population is controversial.Citation28 Several recently published studies have demonstrated that the TNF-alpha −308 AA/GA allele is more prevalent and may be strongly associated with increased risk of CVD.Citation29 Studies investigating possible correlations between cytokine genotypes and CVD in HD patients are rare, but data have been reported for this genotype showing that the A allele seems to be associated with increased risk of complications (). Balakrishnan et al.Citation30 demonstrated that HD patients who were A allele carriers had increased comorbidity and lower serum albumin levels. Similarly, a study of patients with acute renal failure demonstrated an increased risk of death among A allele carriers.Citation31 Ram et al.Citation32 demonstrated an increased risk of synthetic graft thrombosis among HD patients who had the G/A and A/A genotypes. Finally, a recent study performed by Buraczynska et al. showed that the A allele of the TNF-alpha −308 polymorphism is associated with CVD in ESRD patients on HD.Citation33 This study seems to support our findings. To date, no clinical study has evaluated the direct relationship between cytokine genotype and atherosclerosis and left ventricle mass in asymptomatic HD patients. However, the relationship between acute phase reactants/cytokines and cardiovascular mortality and mortality from other causes has been intensively investigated in ESRD and it has been consistently observed that acute phase reactants like serum CRP levelsCitation34,Citation35 and levels of cytokines such as serum TNF-alpha are independently associated with atherosclerosisCitation36. Several studies have also shown that high serum levels of circulating TNF-alpha are possibly involved in the pathogenesis of LVH in HD patients.Citation37 These results agree with our findings demonstrating that differences in inflammatory processes due to genetic differences in the productive capacity of TNF-alpha may play a role in the development of atherosclerosis and LVH in asymptomatic HD patients.
IL-10 is the most important anti-inflammatory and anti-atherogenic immune-regulating cytokine, as it effectively downregulates TNF-alpha, a known proinflammatory cytokine.Citation25 Polymorphic sequences have been described in the IL-10 promoter (−1082 A/G, −819 C/T, −592 C/A) which leads to high or low production of this cytokine. Individuals with homozygous G/G genotypes for the −1082 G allele produce 30% of the cytokine compared with A/G or A/A genotype individuals.Citation10 Recent studies have focused on the relation between genetic variations in the IL-10 gene and CVDs. Patients with unstable angina pectoris had lower serum levels than those with stable angina pectoris, indicating a protective role for the anti-inflammatory cytokine.Citation38 Similarly, cardiovascular protective roles for IL-10 were also reported in dialysis patients. Girndt et al. demonstrated that HD patients with the genotype associated with higher IL-10 levels have a lower risk of cardiovascular death.Citation14 Balakrishnan et al.Citation30 demonstrated that HD patients with the high- and intermediate-producing genotypes (G/G and G/A) had a higher functional score (Karnofsky index) than low producers. Univariate analysis of the relationship between single genotypes of IL-10 and changes in CIMT and LVMI did not reach significance in our study. This could be related to small sample size or a relatively lower prevalence of certain genotypes in this cohort. However, patients with the combination of TNF-alpha high-producer genotypes with the IL-10 low-producer genotype was associated with significantly higher CRP levels and progression of CIMT and LVMI than patients with the low TNF-alpha/high-IL-10 producer genotype combination. Likewise, Balakrishnan et al. also demonstrated that patients with the combination of IL-10 high- or intermediate-producer genotypes with the TNF-alpha low-producer genotype had less comorbidity and higher Karnofsky scores than patients with the opposite polar genotype combinations.Citation30 These observations indicate that synergistic or antagonistic effects may come into play with genotype combinations, and these may have a critical role in susceptibility to chronic inflammation and CVDs in these patients.
Although our results are significant, there were limitations in this study. Small sample size may lead to some bias; meta-analysis of several small and large sample size studies in patients with renal failure will give a clearer idea about genetic associations with CVD in this special patient group. Second, we do not have data from a normal age-matched Turkish cohort. However, a set of Hardy–Weinberg analyses of genotype frequencies reveal that our study group is representative of a population with random mating for the IL-10 −1082, IL-10 −819, IL-10 −592, TNF-alpha −308, and TNF-alpha −238 loci (all p-values are greater than 0.1). Third, we did not measure the serum TNF-alpha and IL-10. Therefore, we were not able to correlate between TNF-alpha −308 A/G and IL-10 −1082 A/G polymorphisms and the serum levels of TNF-alpha and IL-10.
Recent studies have shown a relationship between signs of chronic inflammation, for example, CRP, TNF-alpha, and IL-10 levels, sonographically monitored signs of atherosclerosis, and echocardiographically defined LVH in the general population.Citation20–22,Citation38 However, the number of studies in HD patients is small. Our results suggested that inflammatory processes in the pathogenesis of CVD might be strongly enhanced in HD patients and those genetic differences in the productive capacity of TNF-alpha and IL-10 might influence the risk for CVD in these patients. CVDs remain the most important cause of death in the dialysis population in spite of the development of new treatment modalities. More studies on the effects of cytokines on CVD in patients with renal failure may be important for future pharmacogenetic treatment.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Zimmermann J, Herrlinger S, Pruy A, Metzger T, Wanner C. Inflammation enhances cardiovascular risk and mortality in hemodialysis patients. Kidney Int. 1999;55(2):648–658.
- Nishizawa Y, Shoji T, Maekawa K, Intima-media thickness of carotid artery predicts cardiovascular mortality in hemodialysis patients. Am J Kidney Dis. 2003;41(3 Suppl. 1):76–79.
- Silberberg JS, Barre PE, Prichard SS, Sniderman AD. Impact of left ventricular hypertrophy on survival in end-stage renal disease. Kidney Int. 1989;36(2):286–290.
- Zoccali C, Benedetto FA, Mallamaci F, Inflammation is associated with carotid atherosclerosis in dialysis patients. Creed Investigators. Cardiovascular risk extended evaluation in dialysis patients. J Hypertens. 2000;18(9):1207–1213.
- Wang AY, Wang M, Woo J, Inflammation, residual kidney function, and cardiac hypertrophy are interrelated and combine adversely to enhance mortality and cardiovascular death risk of peritoneal dialysis patients. J Am Soc Nephrol. 2004;15(8):2186–2194.
- Makhatadze NJ. Tumor necrosis factor locus: Genetic organization and biological implications. Hum Immunol. 1998;59(9):571–579.
- Cope AP. Regulation of autoimmunity by proinflammatory cytokines. Curr Opin Immunol. 1998;10(6):669–676.
- Van der Poll T, Jansen J, Levi M, ten Cate H, ten Cate JW, van Deventer SJ. Regulation of interleukin 10 release by tumor necrosis factor in humans and chimpanzees. J Exp Med. 1994;180(5):1985–1988.
- Wilson AG, Symons JA, McDowell TL, McDevitt HO, Duff GW. Effects of a polymorphism in the human tumor necrosis factor alpha promoter on transcriptional activation. Proc Natl Acad Sci USA. 1997;94(7):3195–3199.
- Turner DM, Williams DM, Sankaran D, Lazarus M, Sinnott PJ, Hutchinson IV. An investigation of polymorphism in the interleukin-10 gene promoter. Eur J Immunogenet. 1997;24(1):1–8.
- Koch W, Kastrati A, Böttiger C, Mehilli J, von Beckerath N, Schömig A. Interleukin-10 and tumor necrosis factor gene polymorphisms and risk of coronary artery disease and myocardial infarction. Atherosclerosis. 2001;159(1):137–144.
- Wang XL, Oosterhof J. Tumor necrosis factor alpha G-308 – A polymorphism and risk for coronary artery disease. Clin Sci (Lond). 2000;98(4):435–437.
- Bernard V, Pillois X, Dubus I, The –308 G/A tumor necrosis factor-alpha gene dimorphism: A risk factor for unstable angina. Clin Chem Lab Med. 2003;41(4):511–516.
- Girndt M, Kaul H, Sester U, Anti-inflammatory interleukin-10 genotype protects dialysis patients from cardiovascular events. Kidney Int. 2002;62(3):949–955.
- Lang RM, MD, Bierig M, Devereux RB, Recommendations for chamber quantification: A report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463.
- Kawagishi T, Nishizawa Y, Konishi T, High resolution B-mode ultrasonography in evaluation of atherosclerosis in uremia. Kidney Int. 1995;48:820–826.
- Pascazio L, Bianco F, Giorgini A, Echo color Doppler imaging of carotid vessels in hemodialysis patients: Evidence of high levels of atherosclerotic lesions. Am J Kidney Dis. 2002;28:713–720.
- Friedwald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low density lipoprotein cholesterol in plasma without use of the preparative ultracentrifuge. Clin Chem. 1972;13:499–502.
- Yao Q, Lindholm B, Stenvinkel P. Inflammation as a cause of malnutrition, atherosclerotic cardiovascular disease, and poor outcome in hemodialysis patients. Hemodial Int. 2004;8:118–129.
- Baldassarre D, De Jong A, Amato M, Carotid intima-media thickness and markers of inflammation, endothelial damage and hemostasis. Ann Med. 2008;40(1):21–44.
- Iwashima Y, Horio T, Kamide K, C-reactive protein, left ventricular mass index, and risk of cardiovascular disease in essential hypertension. Hypertens Res. 2007;30(12):1177–1185.
- Mehta SK, Rame JE, Khera A, Left ventricular hypertrophy, subclinical atherosclerosis, and inflammation hypertension. Hypertension. 2007;49(6):1385–1391.
- Stenvinkel P, Pecoits-Filho R, Lindholm B, for the DialGene Consortium. Gene polymorphism association studies in dialysis: The nutrition-inflammation axis. Semin Dial. 2005;18(4):322–330.
- Jeanmonod P, von Känel R, Maly FE, Fischer JE. Elevated plasma C-reactive protein in chronically distressed subjects who carry the A allele of the TNF-alpha –308 G/A polymorphism. Psychosom Med. 2004;66(4):501–506.
- Stenvinkel P, Ketteler M, Johnson RJ, IL-10, IL-6, and TNF-alpha: Central factors in the altered cytokine network of uremia – the good, the bad, and the ugly. Kidney Int. 2005;67(4):1216–1233.
- Abraham LJ, Kroeger KM. Impact of the –308 TNF promoter polymorphism on the transcriptional regulation of the TNF gene: Relevance to disease. J Leukoc Biol. 1999;66(4):562–566.
- Vatay A, Bene L, Kovács A, Relationship between the tumor necrosis factor alpha polymorphism and the serum C-reactive protein levels in inflammatory bowel disease. Immunogenetics. 2003;55(4):247–252.
- Pereira TV, Rudnicki M, Franco RF, Pereira AC, Krieger JE. Effect of the G-308A polymorphism of the tumor necrosis factor alpha gene on the risk of ischemic heart disease and ischemic stroke: A meta-analysis. Am Heart J. 2007;153(5):821–830.
- Elahi M, Gilmour A, Matata BM, Mastana SS. A variant of position -308 of the Tumour necrosis factor alpha gene promoter and the risk of coronary heart disease. Heart Lung Circ. 2008;17(1):14–18.
- Balakrishnan VS, Guo D, Rao M, Cytokine gene polymorphisms in hemodialysis patients: Association with comorbidity, functionality, and serum albumin. Kidney Int. 2004;65:1449–1460.
- Jaber BL, Rao M, Guo D, Cytokine gene promoter polymorphisms and mortality in acute renal failure. Cytokine. 2004;25(5):212–219.
- Ram S, Bass K, Abreo K, Baier RJ, Kruger TE. Tumor necrosis factor-alpha −308 gene polymorphism is associated with synthetic hemodialysis graft failure. J Investig Med. 2003;51(1):19–26.
- Buraczynska M, Mierzicki P, Buraczynska K, Dragan M, Ksiazek A. Tumor necrosis factor-alpha gene polymorphism correlates with cardiovascular disease in patients with end-stage renal disease. Mol Diagn Ther. 2007;11(4):257–263.
- Yeun JY, Levine RA, Mantadilok V, Kaysen GA. C-Reactive protein predicts all-cause and cardiovascular mortality in hemodialysis patients. Am J Kidney Dis. 2000;35(3):469–476.
- Zoccali C, Mallamaci F, Tripepi G. Inflammation and atherosclerosis in end-stage renal disease. Blood Purif. 2003;21(1):29–36.
- Perunicic-Pekovic G, Pljesa S, Rasic-Milutinovic Z, Stankovic S, Ilic M, Maletic R. Inflammatory cytokines and malnutrition as related to risk for cardiovascular disease in hemodialysis patients. Can J Physiol Pharmacol. 2008;86(4):205–209.
- Nishimura M, Hashimoto T, Kobayashi H, Possible involvement of TNF-alpha in left ventricular remodeling in hemodialysis patients. J Nephrol. 2003;16(5):641–649.
- Smith DA, Irving SD, Sheldon J, Cole D, Kaski JC. Serum levels of the anti-inflammatory cytokine interleukin-10 are decreased in patients with unstable angina. Circulation. 2001;104(7):746–749.