Abstract
Aims: To date, there is convincing evidence for the preservation of residual renal function (RRF) in peritoneal dialysis (PD) patients; however, substantially variable data exist on the incidence rate of infectious complications and the decline of RRF for automated peritoneal dialysis (APD) and continuous ambulatory peritoneal dialysis (CAPD). The purpose of our study was to investigate the relative merits or demerits of APD compared with CAPD. Methods: From November 1998 to November 2007, we retrospectively reviewed 32 patients on APD and 140 patients on CAPD. We compared incidences of infectious complications during the entry period. RRF and other PD parameters were determined and compared over 2 years of therapy. In addition, the period of hospitalization was also included for clinical outcome analysis. Results: There were no significant differences between the two modalities with regard to the incidence of peritonitis (1.42/100 patient–months for APD vs. 1.23/100 patient–months for CAPD, p = 0.66). At the end of the second year, there were no significant differences between APD and CAPD with regard to the decline of RRF (14.8 vs. 15.3 L/week/1.73 m2, p = 0.84). However, APD significantly increased the value of total weekly Kt/V during this period. Furthermore, we observed a significant reduction in hospitalized days of APD compared with CAPD. Conclusions: We concluded that the selection of the PD modality is not a major determinant of the decline in RRF. APD can be adapted to the targeted adequacy and is at least as efficacious as CAPD when it is expertly applied.
INTRODUCTION
Continuous ambulatory peritoneal dialysis (CAPD) or automated peritoneal dialysis (APD) can be applied to end-stage renal disease (ESRD) patients who want to be treated with peritoneal dialysis (PD), but there are instances when one form of therapy is preferred over the other. PD has been claimed to provide preservation of residual renal function (RRF) for the majority of dialysis patients,Citation1–3 and RRF is an important predictor of survival among PD patients.Citation4,Citation5 The use of APD has steadily increased during the past decade, mainly driven by the improved design of cyclers and of its ability to be adapted to the patients' individual needs with respect to life style. Given this increasing trend toward the greater use of APD, it is important to know if the proposed clinical benefits of APD are realized when compared to conventional CAPD and whether it is associated with an increased risk of accelerated RRF decline,Citation6,Citation7 or a decreased incidence of peritonitis.Citation8–11 Despite the importance of preserving RRF, there are inconsistent data on the compared ability of CAPD and APD for the preservation of RRF after the initiation of PD therapy. The first purpose of our study was to compare the changes in RRF and infectious complications between APD and CAPD.
In addition to a major role in maintaining water and electrolyte balance and in eliminating so-called middle molecules, RRF is one of the factors that determine adequacy in PD patients, in terms of weekly urea clearance in liters/liters total body water (Kt/V) or creatinine clearance (CrCl) in liters per week per 1.73 m2 body surface area.Citation12,Citation13 However, the consequences of serial differences in total weekly Kt/V or CrCl and the definition of the PD prescription (instilled volume, use of hypertonic dialysates, etc.) applied in APD and in CAPD have not been addressed in the majority of the previous studies. Therefore, these factors have not been assessed in detail so as to disclose their potential effects on clinical outcome. The second purpose of our study was to compare the changes in these parameters between APD and CAPD.
MATERIALS AND METHODS
This retrospective cohort study was performed using a chart review design on 447 patients who consulted the PD institution at a medical center in southern Taiwan between November 1998 and November 2007. The exclusion criteria were as follows: (1) less than 16 years of age; (2) anuria with recent initiation of PD therapy; (3) discontinuation of PD for the following reasons: kidney transplantation, technique failure, death, transfer to hemodialysis, and loss to follow-up; and (4) changes in PD modality during the first or second year of therapy. According to the study protocol, all patients completed at least 2 years of consecutive PD therapy; a total of 172 clinically stable patients (32 on APD and 140 on CAPD) were finally eligible. The comments below relate mainly to APD with continuous cycling PD, which consisted of several cycles (usually 4–5) performed at night, over 9–10 h, and a long daytime dwell. The clinical characteristics for all patients, including demographic and biochemical data, and the PD adequacy index when starting PD therapy, were reviewed for statistical analysis and comparison between APD and CAPD. The incidence of peritonitis, exit-site infections, and tunnel infections was compared. Total infectious complications were defined as the total episodes of peritonitis, exit-site infections, and tunnel infections. RRF was assessed using a 24 h urine CrCl measurement just before the start of PD, at 6 months, 1 year, and 2 years (to convert the unit of measurement of CrCl from mL/min/1.73 m2 to L/week/1.73 m2, it was multiplied by 10.08). A peritoneal equilibration test (PET) was performed 1 month after the initiation of PD and was repeated every 6 months. The days of hospitalization over a 2-year period were compared for clinical outcome analysis.
Statistical analysis
Results are expressed as mean ± SD for continuous data and as frequencies or percentages for categorical data. These values and differences between APD and CAPD were compared using two-tailed unpaired t-test or Mann–Whitney U-test (numerical variables), and χ2-test (categorical variables), as appropriate. The time interval to first peritonitis was analyzed by using Kaplan–Meier survival analysis. The infection incidence was calculated with the help of the Fisher's exact test and χ2-test. A p-value <0.05 was considered statistically significant. All statistics were carried out using SPSS, version 17.
RESULTS
shows the baseline demographic and clinical characteristics of the study population. The primary causes of renal failure were diabetic nephropathy (19/172, 11.0%), glomerulonephritis (125/172, 72.7%), and others (28/172, 16.3%). The patients were predominantly male observed in the APD group (59.0%) compared to the CAPD group (39%). The mean follow-up period was 46.1 months for the APD patients and 49.8 months for the CAPD patients (p = 0.44). Patients in the CAPD group demonstrated higher LDL-cholesterol levels and were prescribed statins more often than those in the APD group.
TABLE 1. Clinical demographic and biochemical data in patients receiving APD and CPAD
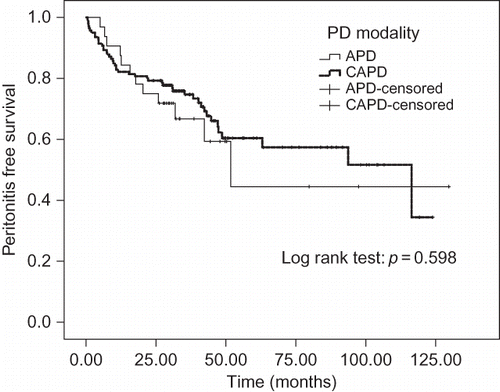
The incidences of peritonitis, exit-site infections, and total infectious complications were not significantly different between the APD and CAPD groups during the entry period. The APD group showed a higher incidence of tunnel infection than the CAPD group (p = 0.04) (). The mean interval to first episode of peritonitis for CAPD (79.1 ± 5.3 months) was longer than that for APD patients (76.0 ± 13.6 months), but the difference was not significant (p = 0.598) (). Consequently, within the 2-year follow-up period, the incidence of new episodes of peritonitis was also similar between the two modalities.
TABLE 2. Incidences of infection complications in patients receiving APD and CAPD
FIGURE 1. Kaplan–Meier survival analysis showed the similar mean interval to first episode of peritonitis for APD and CAPD.
shows the longitudinal changes in urine CrCl, dialysis adequacy parameters, prescription dose, and daily ultrafiltration volume in the two groups. There were no significant differences between APD and CAPD for baseline urine CrCl, PET 4 h dialysate/plasma creatinine ratio (4 h D/P Cr), normalized protein catabolic rate (nPCR), changes in the urine weekly CrCl, and the total weekly CrCl in a 2-year period. There was a significant increase in Kt/V values in the APD group at 6, 12, and 24 months compared to the CAPD group: 0.13 versus –0.18, 0.12 versus –0.06, and 0.16 versus –0.11, respectively. There were no significant differences in the volumes of hypertonic dialysate (3.86% glucose dialysis solution) and ultrafiltration between the two groups; however, the daily dialysate volume was higher in the APD group (p < 0.05).
TABLE 3. Comparison of peritoneal dialysis parameters in APD and CAPD patients at 6 months, 1 year, and 2 years
shows the comparison of the decline of RRF at the 2-year follow-up based on gender. In our study, males had the greatest decline in RRF in the PD group (–20.8 vs. –11.0 L/week/1.73 m2, p < 0.01). Male patients in the APD or CAPD groups had a greater deterioration of RRF when compared with female patients, but the difference was only significant in the CAPD group.
TABLE 4. Comparisons of clinical characteristics based on gender
A total of 104 patients (60.5%) received angiotensin-converting enzyme inhibitors (ACEis) or angiotensin receptor blockers (ARBs) and had a decline in their RRF (–17.1 L/week/1.73 m2) compared with the non-ACEis or ARBs groups (–12.3 L/week/1.73 m2, p = 0.13) at the 2-year follow-up. Fifteen patients (1 on APD, 14 on CAPD) developed peritonitis within 6 months of commencing PD therapy. The decrease in RRF in the first year was more severe for the early-onset peritonitis group (<6 months) than the peritonitis-free group (–17.1 vs. –8.7 L/week/1.73 m2, p = 0.02). In addition, 90 patients (18 on APD; 72 on CAPD) used hypertonic dialysate as a PD prescription within the first 12 months. CrCl decreased 15.5 and 14.8 L/week/1.73 m2 2 years after hypertonic dialysate and standard dialysate treatment, respectively (p = 0.60, data not shown in the table).
shows the number of times of hospitalization and the period of hospitalization in the 2-year period after commencement of PD. The relevant causes of hospitalization in the APD (CAPD) groups were as follows: peritonitis, 6 (19); systemic infections (pneumonia, sepsis, etc.), 3 (10); hernia, 3 (3); cardiovascular disease or stroke, 1 (7); and gastrointestinal or genitourinary bleeding, 2 (6). In the 2-year period, there was no significant difference in the frequency of hospitalization between the APD and CAPD patients; however, APD patients had a lesser average hospitalized days than the CAPD patients (p = 0.03).
TABLE 5. Hospitalizations and hospitalized days within the first 2 years of PD
DISCUSSION
APD is a general term used to describe all types of PD performed with the help of a cycler and serves as the first-line PD treatment to disabled, aged, or active patients. The indications for using APD in our PD institution are patient preference, the necessity to avoid increased intraperitoneal pressure, and an inability to obtain adequate ultrafiltration or solute clearance, especially in high-transporter patients. APD has been reported to have several advantages over CAPD, including a lower incidence of peritonitis, mainly on account of fewer connections during the daytime.Citation8–11,Citation14 However, the evidence with respect to the effect of APD on peritonitis when compared to CAPD is controversial because the majority of these studies were observational studies and hence prone to biases; therefore, their results may not be entirely reliable.Citation15 Our results did not demonstrate the superiority of APD to CAPD in reducing episodes of peritonitis. In other words, we may conclude that selection of the PD modality was not a major determinant of peritonitis incidence. Six patients (three on APD and three on CAPD) developed total eight times of tunnel infection. In particular, these patients did not have any episode of peritonitis, so tunnel infection in APD group maybe not the underlying reason for the equal incidences of peritonitis in APD and CAPD groups. In addition, we could not demonstrate that APD had a real higher tunnel infection incidence because of the low statistic power.
Comparison of the baseline demographic and clinical characteristics showed that the two groups were well matched except that males were more predominant in the APD group than in the CAPD group. This was because the majority of male patients tended to chose APD in our PD institution as it eliminates the need for a manual daytime exchange (even with the long daytime dwell) and, as a consequence, allows them to remain employed. Other demographic and clinical characteristics, including age, causes of ESRD, body mass index, biochemistry, daily urine output, baseline RRF, and peritoneal transport parameters were similar. Therefore, we concluded that any selection bias was minimized.
The gender influences on RRF in PD patients have not been well investigated in previous studies, except by Singhal et al.Citation16 who reported that the male gender and an increased body mass index were predictors of RRF loss. In the present study, we demonstrated that male patients had a faster decline in RRF than female patients in the 2-year follow-up period, and that this phenomenon was significant in the CAPD cohort; however, this result needs to be further investigated. Older patients also have a more rapid decline of RRF when PD is initiated.Citation16,Citation17 In the present study, age was similar between the two groups, and its influence on RRF was minimal.
ACEis and ARBs are widely used in clinical practice to control blood pressure, preserve RRF, and decrease cardiovascular morbidity and mortality in patients with diabetic nephropathy and chronic proteinuric nephropathy.Citation18,Citation19 Limited data indicate that use of ACEis and ARBs may slow the decline of RRF in PD patients.Citation20–22 In the present study, subgroup analysis showed that ACEi or ARB users did not exhibit significant preservation of RRF in comparable groups.
The rate of peritonitis is an independent risk factor for the decline of RRF in PD.Citation23 Moreover, previous studies reported that the early onset of peritonitis during a 6-month entry period is a predictor of the presence of recurrent peritonitis and technique failure, all of which have a detrimental effect on RRF.Citation24,Citation25 Our study, in accordance with these previous investigations, indicated poor RRF outcomes in patients suffering from early-onset peritonitis.
Other factors affecting the preservation of RRF in PD, apart from underlying diseases, gender, peritonitis, or prescribed drugs, have not been assessed in depth or clearly identified. We focused on the effects of the different PD modalities on preservation of RRF. We enrolled clinically stable patients in order to analyze the inherent influence of APD and CAPD on RRF. Although CrCl has been shown to overestimate the glomerular filtration rate (GFR) in ESRD patients because of the increased tubular secretion of creatinine, it is well correlated to inulin clearance and is much simpler to measure.Citation26 We believe that the use of the calculated daily urine CrCl (or conversion to weekly urine CrCl) instead of the estimated GFR is simpler in clinical practice.
Hiroshige et al.Citation6 reported the negative influence of APD on RRF. In their 6 months prospective nonrandomized study, the changes in renal CrCl rates were −0.34 and +0.01 mL/min monthly for patients on APD and CAPD, respectively. Hufnagel et al.Citation7 reported a similar result, and they assumed that the accelerated decline of RRF in their APD group was due to acute changes in osmotic loading and volume removal. De Fijter et al.Citation27 conducted a prospective randomized study and reported no significant difference in the decline of RRF (mean monthly decline in CrCl was 0.07 mL/min in CAPD patients and 0.08 mL/min in APD patients, over a 24-months follow-up period). The major finding of our study is that the serial changes in renal weekly CrCl and total weekly CrCl were similar in both groups at 6 months, 1 year, and 2 years. The volume of 3.86% glucose dialysis solution and ultrafiltration volume were also similar. Greater variations in osmotic load has been reported to further damage RRF in PD patientsCitation7; we therefore analyzed the effect of the hypertonic dialysate on the decline of RRF and did not observe a deterioration of RRF with a hypertonic PD solution.
APD patients had a higher daily dwell volume and weekly Kt/V than CAPD patients. In view of the increased Kt/V in our APD patients, an additional daily exchange was one approach that was used to reach dialysis adequacy. In patients of the high and high-average peritoneal category, it is reasonable to increase the exchanges in the APD prescription. In contrast, increased exchanged volume is more feasible in the low-transport category.Citation28
To date, the published data suggest that the long-term outcomes in patients treated with APD are at least as good as those observed in CAPD patients.Citation29 Our study also demonstrated a similar observation, either with regard to the incidence of infections or the decline of RRF. We also found that APD patients were hospitalized for fewer days than CAPD patients. This result was difficult to interpret from the demographic and dialysis adequacy data of the APD and CAPD patients, and further investigation is warranted to clarify this issue.
In conclusion, our present study revealed that APD and CAPD had similar incidences of infections and decline of RRF over the 2-year observation period. In terms of peritonitis, CAPD did not have a disadvantage compared to APD. APD patients had higher weekly Kt/V values than CAPD patients as a result of the increased dialysate volume. Moreover, APD patients were hospitalized for fewer days compared to the CAPD patients. From the results of present study, we conclude that selection of the PD modality was not a major determinant of the decline of RRF, but rather, the clinical practices employed influenced the decline in RRF. APD is at least as efficacious as CAPD when expertly applied.
Acknowledgments
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this paper.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this paper.
REFERENCES
- Lameire NH. The impact of residual renal function on the adequacy of peritoneal dialysis. Nephron. 1997;77:14–28.
- Moist LM, Port FK, Orzol SM, Predictors of loss of residual renal function among new dialysis patients. J Am Soc Nephrol. 2000;11:556–564.
- Jansen MA, Hart AA, Korevaar JC, Predictors of the rate of decline of residual renal function in incident dialysis patients. Kidney Int. 2002;62:1046–1053.
- CANUSA Peritoneal Dialysis Group. Adequacy of dialysis and nutrition in continuous peritoneal dialysis: Association with clinical outcomes. J Am Soc Nephrol. 1996;7:198–207.
- Bargman JM, Thorpe KE, Churchill DN. For the CANUSA Peritoneal Dialysis Study Group. The importance of residual renal function for survival in patients on peritoneal dialysis. J Am Soc Nephrol. 1997;8:185A (Abstract).
- Hiroshige K, Yuu K, Soejima M, Rapid decline of residual renal function in patients on automated peritoneal dialysis. Perit Dial Int. 1996;16:307–315.
- Hufnagel G, Michel C, Queffeulou G, The influence of automated peritoneal dialysis on the decrease in residual renal function. Nephrol Dial Transplant. 1999;14:1224–1228.
- Holley JL, Bernardini J, Piraino B. Continuous cycling peritoneal dialysis is associated with lower rates of catheter infections than continuous ambulatory peritoneal dialysis. Am J Kidney Dis. 1990;16:133–136.
- Brunkhorst R, Wrenger E, Krautzig S, Clinical experience with home automated peritoneal dialysis. Kidney Int. 1994;46(Suppl. 48):S25–30.
- Korbet SM, Vonesh EF, Firanek CA. Peritonitis in an urban peritoneal dialysis program: An analysis of infecting pathogens. Am J Kidney Dis. 1995;26:47–53.
- Rodríguez-Carmona A, Pérez Fontán M, García Falcón T, A comparative analysis on the incidence of peritonitis and exit-site infection in CAPD and automated peritoneal dialysis. Perit Dial Int. 1999;19:253–258.
- Rodby RA, Firanek CA, Cheng YG, Reproducibility of studies of peritoneal dialysis adequacy. Kidney Int. 1996;50:267–271.
- Bargman JM, Thorpe KE, Churchill DN. Relative contribution of residual renal function and peritoneal clearance to adequacy of dialysis: A reanalysis of the CANUSA study. J Am Soc Nephrol. 2001;12:2158–2162.
- Kieman L, Kliger A, Gorban-Brennan N, Comparison of continuous ambulatory peritoneal dialysis-related infections with different ‘Y-tubing’ exchange systems. J Am Soc Nephrol. 1995;5:1835–1837.
- Rabindranath KS, Adams J, Ali TZ, Automated vs continuous ambulatory peritoneal dialysis: A systematic review of randomized controlled trials. Nephrol Dial Transplant. 2007;22:2991–2998.
- Singhal MK, Bhaskaran S, Vidgen E, Rate of decline of residual renal function in patients on continuous peritoneal dialysis and factors affecting it. Perit Dial Int. 2000;20:429–438.
- Lysaght MJ, Vonesh EF, Gotch F, The influence of dialysis treatment modality on decline of remaining renal function. ASAIO Trans. 1991;37:598–604.
- Brenner BM, Cooper ME, de Zeeuw D, Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869.
- Mora-Macia J, Cases A, Calero F, Effect of angiotensin II receptor blockade on renal disease progression in patients with non-diabetic chronic renal failure. Nephrol Dial Transplant. 2001;16:82–84.
- Li PK, Chow KM, Wong TY, Effects of an angiotensin-converting enzyme inhibitor on residual renal function in patients receiving peritoneal dialysis. A randomized, controlled study. Ann Intern Med. 2003;139:105–112.
- Suzuki H, Kanno Y, Sugahara S, Effects of an angiotensin II receptor blocker, valsartan, on residual renal function in patients on CAPD. Am J Kidney Dis. 2004;43:1056–1064.
- Akbari A, Knoll G, Ferguson D, Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in peritoneal dialysis: Systematic review and meta-analysis of randomized controlled trials. Perit Dial Int. 2009;29:554–561.
- Shin SK, Noh H, Kang SW, Risk factors influencing the decline of residual renal function in continuous ambulatory peritoneal dialysis patients. Perit Dial Int. 1999;19:138–142.
- Port FK, Held PJ, Nolph KD, Risk of peritonitis and technique failure by CAPD connection technique. A national study. Kidney Int. 1992;42:967–974.
- Alexander SR, Sullivan EK, Harmon WE, Maintenance dialysis in North American children and adolescents: A preliminary report. North American Pediatric Renal Transplant Cooperative Study (NAPRTCS). Kidney Int. 1993;44(Suppl. 43):S104–109.
- Lavender S, Hilton PJ, Jones NF. The measurement of glomerular filtration rate in renal disease. Lancet. 1969;2:1216–1219.
- De Fijter CW, Oe LP, Nauta JJ, Clinical efficacy and morbidity associated with continuous cyclic compared with continuous ambulatory peritoneal dialysis. Ann Intern Med. 1994;120:264–271.
- Brunkhorst RR. Individualized PD prescription: APD versus CAPD. Perit Dial Int. 2005;25(Suppl. 3):S92–94.
- Mehrotra R, Chiu YW, Kalantar-Zadeh K, The outcomes of continuous ambulatory and automated peritoneal dialysis are similar. Kidney Int. 2009;76:97–107.
