Abstract
Mesenchymal stem cell (MSC) has been implied to have the therapeutic potential on chronic kidney disease (CKD). However, the underlying mechanism is still unclear and administration frequency of MSCs could be an issue in a chronic disease model. We evaluated the effect of repeated administration of MSCs on a remnant kidney model. MSCs from 5-week male Sprague–Dawley rats were infused by tail vein into 7-week female 5/6 nephrectomized rats after tagging with a fluorescent probe, chloromethyl-1,1-dioctadecyl-3,3,3′,3′-tetramethyl- indocarbocyanine perchlorate (CM-Dil). Effect of weekly administration of MSCs was compared with the effect of once injection of MSCs and mesangial cells (MCs) at 1 and 5 weeks, respectively. Engraftment of MSCs into the kidney was evaluated by the presence of CM-Dil fluorescence or SRY gene expression. Weekly MSCs administration showed significant improvement in systolic blood pressure (SBP), urinary protein excretion amount, and serum creatinine level at 5 weeks, whereas once MSCs or MCs administration did not. Although once MSCs administration attenuated glomerulosclerosis and infiltration of ED-1 positive cells at 5 weeks as compared with MCs, weekly MSC administration led to a more significant improvement. Renal SRY gene expression and presence of CM-Dil-tagged cells could be confirmed at 1 week after injection of MSCs or weekly injected group, but not at 5 weeks after once injection. MSCs attenuated cortical expression of interleukin (IL)-6 and elevated the expression of IL-10, but these effects were only sustained in the weekly group. Thus, repeated administration of MSCs improves the protective effect on remnant kidney injury, but primarily via the paracrine effect rather than differentiation.
INTRODUCTION
The bone marrow (BM) contains at least two kinds of stem cells, hematopoietic stem cells (HSCs), giving rise to all differentiated blood cells, and mesenchymal stem cells or marrow stromal cells (MSCs).Citation1 MSC is typically defined as adherent, fibroblastoid-like cells that differentiate to osteoblasts, adipocytes, and chondrocytes in vitro,Citation2 hold special promise for renal repair, because nephrons are largely of mesenchymal origin. In addition, the potentialities of cell therapy for the recovery or improvements of damaged kidneys are extensively studied in recent years.Citation3–5
In experimental models, the administration of in vitro expanded MSCs protected and reversed acute kidney injury.Citation6,Citation7 Because most of these studies could not provide the evidence of the differentiation of injected MSCs into target cells, it was suggested that the renoprotective effects of MSCs against acute kidney injury were primarily mediated via complex paracrine actions.Citation7
The effects of these cells on the model of chronic kidney disease (CKD) are also reported recently. In a murine model of chronic renal disease, BM-derived mononuclear cells were suggested to reduce or stabilize the rate of decline in creatinine clearance.Citation5,Citation8 Lin-negative BM cells were also reported to reduce chronic kidney injury and improve renal function in a remnant kidney model.Citation9 However, the mechanism involved in renoprotective role of MSCs on CKD is still unclear. Differentiation of MSCs to damaged kidney tissue had been suggested in several models of acute kidney injury and glomerulonephritis.Citation10,Citation11 However, still lacking evidence of differentiation of engrafted cells in a remnant kidney model presumed that mechanism of BM-derived cells on CKD could be also complex paracrine action rather than differentiation.
Recently, MSCs have been reported to have immune-modulation capacity in vitro and in vivo experimental models.Citation12,Citation13 Pleiotropic effects of MSCs could provide another therapeutic potential in the challenge with CKD as well as acute kidney injury. Regardless of the capacity of incorporation into kidney tissue, administration dosage and frequency could be critical for paracrine effects of MSCs. In this study, we evaluated the effect of repeated administration of MSCs on a remnant kidney model to assess the therapeutic efficiency and regulation mechanisms of MSCs in preventing the progression of chronic renal injury.
MATERIALS AND METHODS
Animals and biochemical analysis
Seven-week-old female Sprague–Dawley rats with 180–200 g body weight (Central Research Laboratory, Seoul, Republic of Korea) underwent 5/6 nephrectomy. Rats were divided in four groups. Saline control group: 5/6 nephrectomy with saline administration (n = 8); mesangial cells (MCs) control group: 5/6 nephrectomy with MCs administration (n = 16); once MSC group: 5/6 nephrectomy with once MSCs administration (n = 16); and weekly MSCs group: 5/6 nephrectomy with weekly MSCs administration (n = 8). Each of 3 × 10Citation6 MSCs or MCs were injected through the tail vein using 27-gauge needle 1 day after nephrectomy. Four times of 3 × 10Citation6 MSCs also were repeatedly injected in the weekly interval in weekly MSCs group. All experiments in animals were performed according to the guidelines of the hospital animal research ethics committee. Urine was collected to measure the volume, protein and creatinine in metabolic cages at 1 and 5 weeks. Systolic blood pressure (SBP) was using tail-cuff plethysmography at 1 and 5 weeks. Each of eight rats of saline control, MCs controls, and once MSCs administration were sacrificed at 1 week, immediately after 24-h urine collection and blood sampling. Remaining eight rats of MCs controls, once, and weekly administration groups were sacrificed at 5 weeks. The kidneys were removed for histology, Y-chromosome examination via PCR and fluorescent positive cells in confocal microscope.
Isolation, characterization, and in vitro differentiation of MSCs
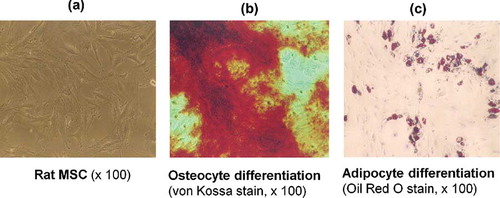
BM-derived MSCs was obtained from 5-week male Sprague–Dawley rats. Briefly, BM was flushed from the shaft of the bone with Dulbecco's modified Eagle's medium (DMEM) (Sigma, St. Louis, MO, USA) containing 10% fetal bovine serum (FBS) plus penicillin/streptomycin (100 U/mL–0.1 mg/mL, GibcoBRL, NY, USA). Adherent cells were obtained by trypsin–ethylenediaminetetraacetic acid (EDTA) (0.5–0.2 g/L; Invitrogen, California, USA), washed with phosphate-buffered saline (PBS), and reseeded at 5 × 10Citation7 cells/cmCitation2. CD14− CD45− β1-integrin+ CD54+ MSCs were confirmed by flow-cytometry. Antibodies directed against β1-integrin, CD14, CD45, and CD54 were labeled according to the manufacturer's protocols (Chemicon, California, USA). The MSCs phenotype was also confirmed by differentiation into osteocytes and adipocytes with specific differentiation media (). Cells on fifth passage were used for experiments. MCs were also cultured and characterized from 5-week male Sprague–Dawley rats as described previously.Citation14
FIGURE 1. Mesenchymal stem cells (MSCs) were characterized by their canonical ability to differentiate into adipocytes and osteocytes. (a) MSCs on second passage growing in spindle-shaped morphology (rat MSCs ×100). (b) MSC cultured for 8 days with osteogenic supplements (von Kossa stain ×100). Osteogenic differentiation is shown by the formation of calcium-hydroxyapatite-positive area (von Kossa staining). (c) MSC cultured for 12 days with adipogenic supplements (Oil Red O stain ×100). Adipogenic differentiation is visualization by Oil Red O staining for intracellular lipid vacuoles.
Fluorescence labeling of MSCs and MCs
Before in vivo injection, cells were labeled using the chloromethyl-1,1-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (CM-Dil, Invitrogen) according to the manufacturer's protocol. CM-Dil-labeling cells were also subcultured to confirm vitality.
Renal histology and immunohistochemical analysis
Tissues were fixed overnight in 10% formalin, dehydrated in alcohol, and then embedded in paraffin. The sections (4 μm thick) were stained with periodic acid Schiff (PAS) reagent or Masson's trichrome (MT). In addition, glomerulosclerosis and inflammation/fibrosis were scored and compared as described previously.Citation15,Citation16 Briefly, glomerulosclerosis index was scored by the mean in 100 glomeruli on a scale from 0 to 3 (0 = normal; 1 < 25%; 2 < 50%; 3 > 50% of the glomerular area per cross section). A semiquantitative evaluation of interstitial inflammation/fibrosis was also scored as the mean of the 10-high powered field. The scores for each of these indicators ranged from 0 to 3+ as follows: 0, no change; 1+, damage to <25% of the interstitial area; 2+, damage to 25–50% of the interstitial area; 3+, damage to >50% of the interstitial area. Immunohistochemistry for ED-1 (rat homologue of human CD68, Serotec, Oxford, UK) was performed on serial sections using an avidin–biotin–peroxidase technique. Quantitative analysis of ED-1-positive cells in glomeruli was performed under 400× magnifications and expressed as the cells/glomerular cross section (gcs). For each section, 50 sequential glomerular profiles were examined. ED-1-positive cells in tubulointerstitium were counted in 25 consecutive high power (×400) interstitial fields by a 0.02 mmCitation2 graticule fitted in the eyepiece of the microscope and expressed as cells/mmCitation2.Citation17
Total RNA isolation and RT-PCR analysis
Total RNA was extracted from cortex and cDNA was synthesized with a reverse transcriptase reaction carried out with the use of standard techniques. RT-PCR was performed in a final volume of 20 μL containing 2 μL of cDNA, 1 μM of each sense and antisense primer (), and 4.4 μL master mix of the Lightcycler FastStart DNA MasterPLUS SYBR Green I kit (Roche Applied Science, Mannheim, Germany). The expression levels were calculated using the ΔCT (threshold cycle) method. Cyclophilin was amplified to control cDNA quantity.
TABLE 1. Sequences of primers for real-time PCR
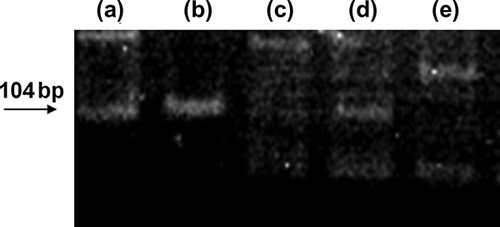
The presence of the sex determination region on the Y-chromosome male (SRY) gene in the recipient female rats was assessed by PCR. Primer sequences for SRY gene (forward 5′-CATCGAAGGGTTAAAGTGCCA-3′, reverse 5′-ATAGTGTGTAG-GTTGTTGTCC-3′) were obtained from published sequences.Citation18
Detection of CM-Dil-specific fluorescence
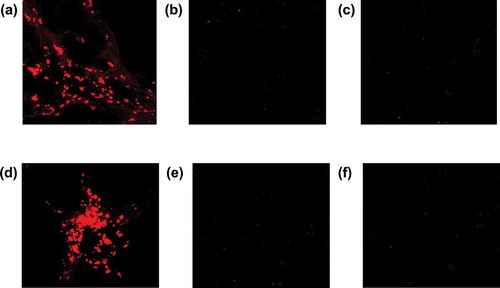
Frozen kidney samples were cut into 5-μm sections and covered using immumount (ThermoShandon, Pittsburgh, PA, USA). Specific fluorescence was analyzed using laser scanning microscopy (LSM 510 META confocal scanning laser microscope; Carl Zeiss, Jena, Germany).
Statistical analyses
All values were expressed as mean ± SD. The changes of BP, urine protein, and serum creatinine from baseline were analyzed by paired Wilcoxon test. Differences among saline control, MCs control, and each MSCs treated groups were evaluated by the nonparametric Kruskal–Wallis test and Dunns post-tests. Differences between once and weekly MSCs-treated groups were evaluated by Mann–Whitney test. Differences yielding p < 0.05 were considered statistically significant.
RESULTS
Change of SBP, urine protein, and serum creatinine
At 5 weeks after 5/6 nephrectomy, SBP was significantly increased in saline and MCs control groups, not in MSCs-treated groups (p < 0.05) (). At 5 weeks, SBP of once MSCs was not different from those of the saline control and MCs control group. However, weekly MSCs group revealed significant lower SBP than those of saline and MCs controls (p < 0.05). Urine protein excretion was also significantly increased at 5 weeks in controls and once MSCs-treated group, but not in weekly MSCs-treated group (p < 0.05). Urine protein excretion of weekly MSCs group (17.1 ± 5.5 mg/day) was significantly lower than those of saline control (22.8 ± 6.7 mg/day) and MCs control (23.9 ± 6.4 mg/day) (p < 0.05). Serum creatinine of weekly MSCs group (0.45 ± 0.08 mg/dL) was also lower than those of saline control (0.55 ± 0.06 mg/dL) and MCs (0.58 ± 0.11 mg/dL) at 5 weeks (p < 0.05).
TABLE 2. Systolic blood pressure, urine volume, and urine protein excretion in studied animals
Renal histological improvement after MSCs injection
Sclerotic change of glomeruli, marked tubular dilatation with interstitial inflammatory cell infiltration and patch fibrosis were characterized in saline and MCs control at 5 weeks of the remnant kidney model. Both of once and weekly MSCs group revealed the reduced sclerotic change of glomeruli, infiltration of inflammatory cells and interstitial fibrosis at 5 weeks. However, weekly MSCs administration led more significant improvement as compared with once MSCs injection ( and ).
FIGURE 2. Renal histology in remnant kidney rats about glomerular sclerosis between the groups after 5 weeks. (a) Saline control group, (b) MCs control group, (c) once MSCs group, and (d) weekly MSCs group. Magnification ×100 (PAS staining). *p < 0.05 as compared with saline and MCs, **p < 0.05 as compared with once MSCs.
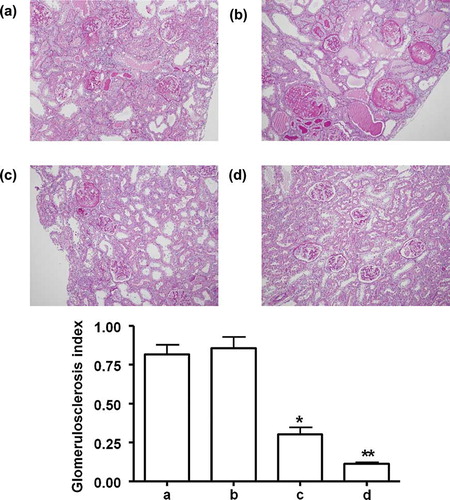
FIGURE 3. Renal histology in remnant kidney rats about glomerular and interstitial fibrosis between the groups after 5 weeks. (a) Saline control group, (b) MCs control group, (c) once MSCs group, and (d) weekly MSCs group. Magnification ×100 (Masson's Trichrome staining). *p < 0.05 as compared with saline and MCs, *p < 0.05 as compared with once MSCs.
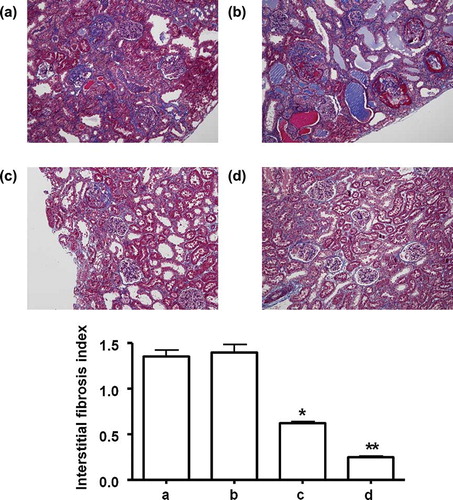
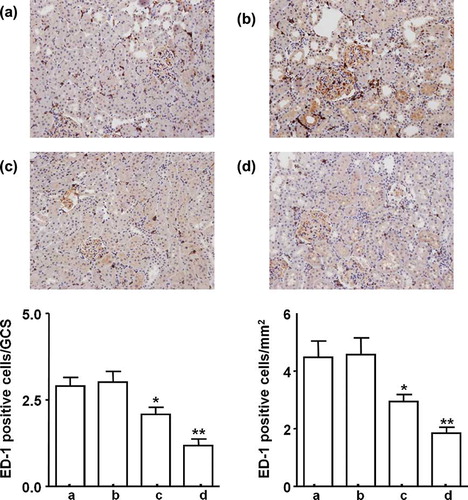
Both of once and weekly MSCs injection significantly decrease interstitial ED-1 positive cell infiltration; however, weekly MSCs administration led more significant attenuation as compared with once MSCs injection ().
FIGURE 4. Infiltration of ED-1 positive cells between the groups after 5 weeks. (a) Saline control group, (b) MCs control group, (c) once MSCs group, and (d) weekly MSCs group. Magnification ×100 (Immunohistochemical staining with anti-ED-1 antibody). *p < 0.05 as compared with saline and MCs control. **p < 0.05 as compared with once MSCs.
Engraftment of MSCs into kidney tissue
The presence of male gender-specific gene could be the indirect evidence of the entrapment of infused donor MSCs into female kidney in this model. Cortical SRY gene was expressed at 1 week after MSCs injection, but not in MCs controls. SRY gene expression was also confirmed in weekly MSCs-treated group but not in once MSCs group at 5 weeks (). Presence of CM-Dil-tagged cells in the renal cortical parenchyma was also confirmed at 1 week after MSCs administration and at 5 weeks in the weekly MSCs administration group (). However, CM-Dil-tagged cell was not found at 5 weeks in once MSCs administration group.
Cytokine gene expression in the renal cortex
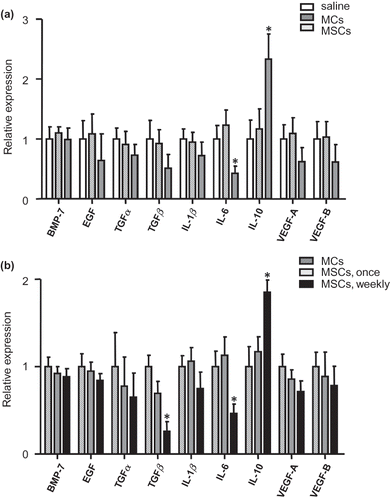
Cortical interleukin (IL)-6 expression was significantly decreased and IL-10 was increased in MSCs administrated rats at 1 week as compared with saline and MCs control (, p < 0.05); however, it could not be maintained to 5 weeks after once MSCs administration. At 5 weeks, cortical transforming growth factor (TGF)-β1 and IL-6 expressions were significantly decreased and IL-10 was increased in weekly MSCs treated group as compared with once MSCs or MCs administration groups (, p < 0.05).
DISCUSSION
Although MSCs may have therapeutic implications in the several models of CKD, evidence of the effect of MSCs on prevention of progression or regeneration from the fibrotic tissue in CKD is still lacking. Remnant kidney model is associated with hypertension, proteinuria, and glomerular sclerosis in histology.Citation15 Systemic and glomerular hypertension was readily observed 30 days after 5/6 nephrectomy rats in association with renal hypertrophy.Citation16 Our study also indicates that 5-week 5/6 nephrectomy model is relevant to study the effect of MSCs on the development of CKD.
In this study, weekly administration of MSCs, but not once MSCs, ameliorated functional parameters including SBP, degree of proteinuria and serum creatinine levels at 5 weeks after 5/6 nephrectomy. In addition, weekly MSC administration led more significant histological improvement as compared with once MSCs injection. Our results showed that administration of MSC had a preventive effect on the development of CKD in a remnant kidney model. There were several conflicting results about the effect of MSCs on models of CKD. Ninichuk et al.Citation8 reported that MSCs reduced interstitial fibrosis in collagen4A3-deficienct mice. Favorable effects of MSCs were also suggested in a remnant kidney model.Citation5,Citation19 However, those studies using a remnant kidney model had not shown any difference in histological improvement between MSCs and control groups.Citation5,Citation19 Recently, Alexandre et al. reported that other BM derived premature cells, Lin− BM cells, could lead histological improvement as well as clinical parameters during the development of chronic renal disease in a remnant kidney model.Citation9 These conflicting results might be due to heterogeneity of stem cells and differences of the route, time and amount of MSCs administration, and from the degree and duration of renal ablation.
BM-derived stem cells to the renal tubule regeneration is still a matter of debate. Several studies showed the administration of MSCs could accelerate the structural recovery from acute kidney injury through amelioration of inflammatory, vascular, and apoptotic/necrotic manifestations.Citation4,Citation6 However, recent data suggested that in vivo differentiation of BM-derived cells into renal tubular cells may not occur at all or is at most a minor component of the repair process.Citation20 In our study, SRY gene expression and CM-Dil-stained cells were found in the renal parenchyma at 1 week after injection of MSCs, but not in MCs administration. These results suggest that MSCs have a homing capacity to injured kidney in a remnant kidney model. However, CM-Dil-stained MSCs and SRY gene expression was not found at 5 weeks after once the administration in this study. It indicates that there was no evidence of differentiation of the implanted MSCs into kidney tissue. And there was no study, which proved the engraftment and differentiation into kidney tissue of infused MSCs in models of CKD. So we could hypothesize that main therapeutic effect of MSCs on CKD might be via paracrine secretion or stimulation of intrarenal progenitor cells.
Several paracrine factors from MSCs were suggested to be involved in renal repair mechanism in a murine model.Citation7,Citation21 At first, vascular endothelial growth factor (VEGF) was suggested as one of the critical paracrine factors, because VEGF regulates angiogenesis and plays an important role in capillary repair of damaged glomeruli.Citation22 Kunter et al.Citation21 suggested that MSCs exerted these therapeutic effects in glomeruli by paracrine effects, such as the release of VEGF and TGF-β1 in the experimental model of glomerulonephritis. However, it has been demonstrated that VEGF mediates glomerular hypertrophy and proteinuria in the model of renal ablation.Citation23 In addition, TGF-β1 was also a well-known mediator of renal fibrosis in a renal ablation model.Citation24 So these cytokines were not regarded as main paracrine factors from MSCs in terms of prevention of the development of chronic injury.
In this study, expression of IL-6 was decreased and IL-10 was increased after administration of MSCs and maintained only in the weekly treated group. In contrast, cortical TGF-β1 expression was decreased in the weekly MSCs injection group in agreement with the improvement of histological findings.
Besides its differentiation and repair property, MSCs were suggested to have pleiotropic effects including immune-modulating capacity. Immunologic activation as well as renin–angiotensin systems suggested being involved in the development of glomerulosclerosis in the remnant kidney model.Citation25 IL-10 is a well-known inhibitor of T cell proliferation and proinflammatory cytokine production.Citation26 MSCs transplantation also modulated the immune-inflammatory response in myocardiac infarction by increased the expression of IL-10.Citation27 The role of immune-regulatory effect of MSCs on the development of CKD was not extensively studied. However, Crop et al.Citation28 showed that the immunosuppressive effect of MSCs was dose-dependent and mediated by IL-10 and indoleamine 2,3-deoxygenase. Up-regulation of IL-10 and attenuation of infiltration of ED-1 positive cells by the administration of MSCs in this study also support that up-regulation of IL-10 expression by MSC might play a role in preventing kidney injury. If pleiotropic effects would be a main mechanism of MSCs on the several kidney disease models, dose and administration frequency of MSCs could be an issue in a chronic injury model such as CKD. Alexandre et al.Citation9 showed that there was no difference between a single infusion and three infusions in 15-day intervals and insisted that the number of did not alter the effect of Lin− cells on glomerulosclerosis. However, our study suggested showed that weekly administration of MSCs could be more effective as compared with once an infusion in prevention of the progression of a renal ablation model. Recently, Cavaglieri et al.Citation29 suggested subcapsular implantation of MSC could be more effective in delivery into injured site and reduction of glomerulosclerosis in this model. These results indicate that regardless of the capacity of incorporation into kidney tissue, administration dosage and frequency could be critical for paracrine effects of MSCs in CKD model. In summary, our experiment showed the weekly administration of MSC improved renal histology and function in 5-week of a remnant kidney model. However, the beneficial effect may be primarily mediated via paracrine effect or stimulation of renal progenitor cells rather than differentiation to kidney tissue.
Acknowledgment
This work was supported by a grant from the Korean Research Foundation (E00138).
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this paper.
REFERENCES
- Pittenger MF, Mackay AM, Beck SC, Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147.
- Dominici M, Le Blank K, Mueller I, Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317.
- Morigi M, Introna M, Imberti B, Human bone marrow mesenchymal stem cells accelerate recovery of acute renal injury and prolong survival in mice. Stem Cells. 2008;26:2075–2082.
- Qian H, Yang H, Xu W, Bone marrow mesenchymal stem cells ameliorate rat acute renal failure by differentiation into renal tubular epithelial-like cells. Int J Mol Med. 2008;22:325–332.
- Choi S, Park M, Kim J, Hwang S, Park S, Lee Y. The role of mesenchymal stem cells in the functional improvement of chronic renal failure. Stem Cells Dev. 2009;18:521–529.
- Morigi M, Imberti B, Zoja C, Mesenchymal stem cells are renotropic, helping to repair the kidney and improve function in acute renal failure. J Am Soc Nephrol. 2004;15:1759–1804.
- Tögel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol. 2005;289:F31–F42.
- Ninichuk V, Gross O, Segerer S, Multipotent mesenchymal stem cells reduce interstitial fibrosis but do not delay progression of chronic kidney disease in collagen4A3-deficient mice. Kidney Int. 2007;70:121–129.
- Alexandre CS, Volpini RA, Shimizu MH, Lineage-negative bone marrow cells protect against chronic renal failure. Stem Cells. 2009;27:682–692.
- Herrera MB, Bussolati B, Bruno S, Fonsato V, Romanazzi GM, Camussi G. Mesenchymal stem cells contribute to the renal repair of acute tubular epithelial injury. Int J Mol Med. 2004;14:1035–1041.
- Striker GE, Mannik M, Tung MY. Role of marrow-derived monocytes and mesangial cells in removal of immune complexes from renal glomeruli. J Exp Med. 1979;149:127–136.
- Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822.
- Inoue S, Popp FC, Koehl GE, Immunomodulatory effects of mesenchymal stem cells in a rat organ transplant model. Transplantation. 2006;81:1589–1595.
- Striker GE, Striker LJ. Glomerular cell culture. Lab Invest. 1986;53:181–191.
- Podjarny E, Bernheim J, Hasdan G, Additive renoprotective effect of candesartan and tetrahydrobiopterin in rats after 5/6 nephrectomy. Nephrol Dial Transplant. 2007;22:1864–1872.
- Fujihara CK, Antunes GR, Mattar AL, Malheiros DM, Vieira JM Jr, Zatz R. Chronic inhibition of nuclear factor-kappa B attenuates renal injury in the 5/6 renal ablation model. Am J Physiol Renal Physiol. 2006;292:F92–F99.
- Jeong KH, Lee TW, Ihm CG, Lee SH, Moon JY, Lim SJ. Effects of sildenafil on oxidative and inflammatory injuries of the kidney in streptozotocin-induced diabetic rats. Am J Nephrol. 2009;29:274–282.
- Rossi P, Dolci S, Albanesi C, Grimaldi P, Geremia R. Direct evidence that the mouse sex-determining gene Sry is expressed in the somatic cells of male fetal gonads and in the germ cell line in the adult testis. Mol Reprod Dev. 1993;34:369–373.
- Caldas HC, Fernandes IM, Gerbi F, Effect of whole bone marrow cell infusion in the progression of experimental chronic renal failure. Transplant Proc. 2008;40:853–855.
- Hishikawa K, Fujita T. Stem cells and kidney disease. Hypertens Res. 2006;29:745–749.
- Kunter U, Rong S, Djuric Z, Transplanted mesenchymal stem cells accelerate glomerular healing in experimental glomerulonephritis. J Am Soc Nephrol. 2006;17:2202–2212.
- Ostendorf T, Kunter U, Eitner F, VEGF-β mediates glomerular endothelial repair. J Clin Invest. 1999;104:913–923.
- Schrijvers BF, Flyvbjerg A, Tilton RG, Rasch R, Lameire NH, De Vriese AS. Pathophysiological role of vascular endothelial growth factor in the remnant kidney. Nephron Exp Nephrol. 2005;101:e9–e15.
- Coimbra TM, Carvalho J, Fattori A, Da Silva CG, Lachat JJ. Transforming growth factor-beta production during the development of renal fibrosis in rats with subtotal renal ablation. Int J Exp Pathol. 1996;77:167–173.
- Hamar P, Peti-Peterdi J, Rázga Z, Kovács G, Heemann U, Rosivall L. Coinhibition of immune and renin-angiotensin systems reduces the pace of glomerulosclerosis in the rat remnant kidney. J Am Soc Nephrol. 1999;10:S234–S238.
- Choi JJ, Yoo SA, Park SJ, Mesenchymal stem cells overexpressing interleukin-10 attenuate collagen-induced arthritis in mice. Clin Exp Immunol. 2008;153:269–276.
- Du YY, Zhou SH, Zhou T, Immuno-inflammatory regulation effect of mesenchymal stem cell transplantation in a rat model of myocardial infarction. Cytotherapy. 2008;10:469–478.
- Crop MJ, Baan CC, Korevaar SS, Donor-derived mesenchymal stem cells suppress alloreactivity of kidney transplant patients. Transplantation. 2009;87:896–906.
- Cavaglieri RC, Martini D, Sogayar MC, Noronha IL. Mesenchymal stem cells delivered at the subcapsule of the kidney ameliorate renal disease in the rat remnant kidney model. Transplant Proc. 2009;41:947–951.