Abstract
Chronic kidney disease (CKD) patients develop anemia because of the low kidney erythropoietin (EPO) production, thus promoting cardiovascular complications. The degree of renal insufficiency might determine the moment to start recombinant human erythropoietin (rhEPO) therapy, but the molecular basis for these options deserves better elucidation. This study aimed to clarify the cardio-renal effects of earlier rhEPO therapy in rats with moderate chronic renal failure (CRF). Four groups of rats were evaluated for 15 weeks (control; rhEPO − 50 IU/kg/week; CRF − 3/4 nephrectomy; CRF + rhEPO) to assess renal and hematology data, EPO levels, blood pressure, heart rate, peripheral catecholamines contents, serum-transforming growth factor-β1 (TGF-β1), kidney gene expression of EPO, Caspase 9 (Casp9), and vascular endothelial growth factor (Vegf). This model of moderate CRF showed moderate and corrected anemia, hypertension, tachycardia, sympathetic overactivity, and increased serum TGF-β1 content. The remnant kidney showed a proliferative profile, with hypertrophy, downregulated gene expression of EPO, and upregulated gene expression of Vegf and Casp9. rhEPO treatment promoted erythrocytosis and prevented tachycardia and catecholamines increment, with a rise of serum TGF-β1. Furthermore, the decreased kidney gene expression of EPO and the overexpression of Casp9 were prevented, demonstrating a renoprotective action on the remnant kidney. In conclusion, rhEPO therapy promotes a protective effect on the cardio-renal axis, which might be mainly attributed to its pro-proliferative and anti-apoptotic properties. These findings might recommend its use in earlier stages of CRF, acting as an erythropoiesis stimulating agent, to efficiently correct not only the anemia, one of the major complications in these patients, but also the succeeding adverse cardio-renal effects.
INTRODUCTION
One of the main side effects of chronic kidney disease (CKD) is anemia development, a frequent complication associated with renal failure, which is mainly because of insufficient erythropoietin (EPO) renal production,Citation1,Citation2 as a result of impaired erythrocytosis mechanisms in the kidney with reduced functional nephrons.Citation3 This anemia further causes cardiac impairment, demonstrated by the increase in cardiac output, left ventricular hypertrophy, congestive heart disease, fatigue, and reduction in exercise capacity.Citation4,Citation5 This triad, already known as cardio-renal anemia syndrome, is a complex pathophysiological entity responsible for the high morbidity and mortality rates found in CKD patients.Citation6–8
The introduction of recombinant human erythropoietin (rhEPO) therapy allowed a significant reduction of anemia-associated adverse effects, allowing for a prolonged life expectancy in end-stage renal disease.Citation9 Apart from the anemia correction, rhEPO therapy has been associated with positive beneficial effects on nonhematopoietic cells,Citation10,Citation11 which have been attributed to its anti-apoptotic, anti-inflammatory, and antioxidant actions that underlie the cardio and neuroprotection in other conditions.Citation12–14 In this context, earlier rhEPO therapy in anemic CKD patients might have a positive cardio-renal impact, such as previously reported in ischemic injury, contributing to organ protection/regeneration.Citation15–17 The degree of renal insufficiency might determine the moment to start rhEPO therapy, but the cellular/molecular basis for these options deserves better elucidation. Considering that renal deterioration or its recovery after pharmacological treatment might be associated with mechanisms of apoptosis, proliferation, and angiogenesis, transforming growth factor-β1 (TGF-β1), vascular endothelial growth factor (Vegf), and Caspase 9 (Casp9) might be useful markers for the elucidation of the contribution of those pathways. Actually, TGF-β1, one of the most important cytokine for the induction of matrix synthesis in the kidney, Vegf, a key marker for vascular endothelial cell differentiation, proliferation, and survival, and Casp9, as part of the apoptosis machinery, are viewed as relevant players to assess the beneficial effect of growth factors in the remnant kidney.
Hypothesizing that renal failure deterioration and cardiovascular events might be better prevented or delayed if CKD patients are earlier identified and treated with rhEPO, this study intended to evaluate, using an animal model of moderate chronic renal failure (CRF) previously characterized,Citation18 the putative cardio-renal benefits of earlier rhEPO therapy.
MATERIALS AND METHODS
Animals and protocol
Male Wistar rats (Charles River Lab., Barcelona, Spain), 250–300 g, were maintained in an air-conditioned room, subjected to 12 h dark/light cycles, and given standard laboratory rat chow (IPM-R20, Letica, Barcelona, Spain) and free access to tap water. Animal experiments were conducted according to the European Communities Council Directives on Animal Care.
The rats were divided into five groups (seven rats each): control − without drugs and surgery; rhEPO (beta) − 50 IU/kg/week s.c. Recormon® (Roche Pharmaceuticals, Welwyn Garden City, UK), without surgery; CRF − induced by a two-stage (3/4) nephrectomy: first, about half of the left kidney was removed and, 1 week later, the entire right kidney was removed; CRF + rhEPO − treated with rhEPO after the third week of surgery; sham-operated − without kidney mass reduction or rhEPO treatment. All the animals have completed a 15-week protocol. Body weight (BW) was monitored during the study and blood pressure (BP) and heart rate (HR) were measured, using a tail-cuff sphygmomanometer LE 5001 (Letica).
Sample collection and preparation
Blood
At the beginning of the experiments and at 3, 9, and 12 weeks after the surgical partial nephrectomy, the rats were subjected to intraperitoneal anesthesia with a 2 mg/kg BW of a 2:1 (v:v) 50 mg/mL ketamine (Ketalar®, Parke-Davis, Lab. Pfeizer Lda, Seixal, Portugal) solution in 2.5% chlorpromazine (Largactil®, Rhône-Poulenc Rorer, Lab. Vitória, Amadora, Portugal). Blood samples were collected from the jugular vein into syringes without anticoagulant (to obtain serum) or with EDTA (for hematological data). To maintain a normal volemia, thus ensuring that results were not changed by the amount of blood collected, some parameters were analyzed only at the final time (15 weeks). A 10 mL blood sample was collected from rats under the anesthesia conditions, above mentioned, to assess circulating catecholamines contents and inflammatory and redox status markers. In the earlier stages, only 2 mL of blood was collected.
Tissues
The rats were sacrificed by cervical dislocation and the heart, adrenals, and kidneys were removed, placed in ice-cold Krebs' buffer and cleaned. The body weight (BW) and the weights of the heart (HW) and kidney (KW), which is only the ¼ remnant kidney for the CKD and CKD + rhEPO groups, were measured to be used to calculate the trophy indexes (HW/BW and KW/BW).
Renal and hematological data
Serum creatinine, urea, and uric acid concentrations were used as renal function indexes through a Hitachi 717 analyzer. Red blood cell (RBC) count, hematocrit (HCT), hemoglobin (Hb) concentration were assessed in whole blood EDTA (Coulter Counter®, Beckman Coulter, Inc., Fullerton, California, USA). Serum EPO concentration and TGF-β1 were measured by ultrasensitive ELISA kits (R&D Systems, Minneapolis, Minnesota, USA).
Peripheral sympathetic nervous system activity
Norepinephrine (NE) and epinephrine (E) concentrations in plasma, platelet, adrenals, and LV were evaluated by high-performance liquid chromatography with electrochemical detection (HPLC-ED),Citation19 using appropriate standards (Sigma Chemical Co., St. Louis, Missouri, USA) and software Gilson 710 (Gilson Software Solutions, Fort Lauderdale, Florida, USA).
Kidney gene expression analysis
Total RNA isolation
Kidneys were isolated in autopsy and stored in RNA later™ solution (Ambion, Austin, Texas, USA). Samples were removed from preservation solution and 1200 μL of RLT Lysis Buffer was added to proceed with disruption and homogenization for 2 min at 30 Hz using TissueLyser (Qiagen, Hilden, Germany). Tissue lysate were processed according to the protocol from RNeasy® Mini Kit (Qiagen). Total RNA was eluted in 50 μL of RNase-free water (without optional treatment with DNAse). To quantify the amount of total RNA extracted and verify RNA integrity (RIN, RNA integrity number), samples were analyzed using 6000 Nano Chip® kit, in Agilent 2100 bioanalyzer (Agilent Technologies, Walbronn, Germany) and 2100 expert software, following manufacturer's instructions. The yield from isolation was from 0.5 to 3 μg; RIN values were 6.0–9.0, and purity (A260/A280) was 1.8–2.0.
Reverse transcription
RNA was reverse transcribed with SuperScript™ III First-Strand Synthesis System for RT-PCR (Invitrogen Corp., Carlsbad, California, USA). One microgram of total RNA was mixed with a 2X First-Strand Reaction Mix and a SuperScript™ III Enzyme Mix (oligo(dT) plus random hexamers). Reactions were carried out in a thermocycler Gene Amp PCR System 9600 (Perkin Elmer, Norwalk, Connecticut, USA), 10 min at 25°C, 50 min at 50°C, and 5 min at 85°C. Reaction products were then digested with 1 μL RNase H for 20 min at 37°C and, finally, cDNA eluted to a final volume of 100 μL and stored at −20°C.
Relative quantification of gene expression
Relative quantification of gene expression was performed using 7900 HT Sequence Detection System (Applied Biosystems, Foster City, California, USA). A normalization step preceded the gene expression quantification, using geNorm Housekeeping Gene Selection kit for Rattus norvegicus (Primer Design, Southampton, UK) and geNorm software (Ghent University Hospital, Center for Medical Genetics, Ghent, Belgium) to select optimal housekeeping genes for this study.Citation20 RT-PCR reactions used optimized specific primers (Proligo, Boulder, Colorado, USA) for genes of interest (), EPO, Casp9, Vegf, and endogenous controls Actb, Gapdh, Top1 together with QuantiTect SYBR Green PCR Kit (Qiagen) Gene expression. RT-PCR reactions were carried out with 100 ng cDNA sample, primers (50–200 nM), and 1× QuantiTect SYBR Green PCR Master Mix. Nontemplate control reactions were performed for each gene, to assure no unspecific amplification. Reactions were performed with the following thermal profile: 10 min at 95°C plus 40 cycles of 15 s at 95°C and 1 min at 60°C. Real-time PCR results were analyzed with SDS 2.1 software (Applied Biosystems) and quantification used the 2−ΔΔCt method.Citation21
Table 1. Genes of interest and endogenous controls used in the present RT-qPCR study
Statistical analysis
For statistical analysis, we used the Statview 4.53 software from Abacus Concepts Inc. (Berkeley, California, USA). Results are presented as means ± standard error of means (SEM). Comparisons between groups and between different times of evaluation were performed using one-way ANOVA and Fisher's test. Significance was accepted at p < 0.05.
RESULTS
The sham-operated animal did not suffer any relevant change concerning the parameters under evaluation and, thus, the data were excluded from the figures and tables to facilitate the interpretation of the results.
Renal function and hematological data
The CRF rats showed a significant increase in serum creatinine and urea concentrations 3 weeks after surgery (data not shown). This increase in renal function markers remained high along the following 12 weeks (). We found that there was a significant decrease (p < 0.05) in creatinine values 12 weeks after surgery in the CRF + rhEPO group versus the CRF group without rhEPO. No significant changes were found for uric acid serum content. Concerning the hematological data, 3 weeks after nephrectomy, the CRF animals showed a statistically significant decrease for RBC count, HTC, and Hb. At week 9, the CRF + rhEPO presented the most prominent changes, with increased RBCs and HCT and Hb values. The CRF group showed the same changes, though with a lower increase. At the end of the protocol (15th week), all the groups recovered the control values. At the same time, serum EPO levels were higher (p < 0.05) in rhEPO rats versus control. A trend to lower serum EPO levels was obtained in CRF rats versus the control. The rhEPO treatment in the CRF animals showed values similar to those of the control ().
Table 2. Effects of rhEPO treatment in renal and hematological data in a rat model of moderate CRF
Blood pressure, heart rate, and tissue trophism indexes
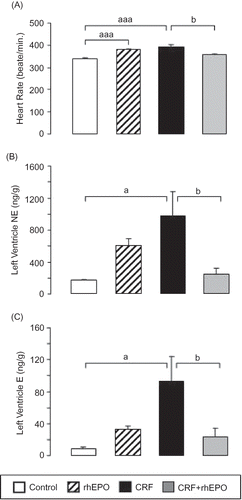
A significant increase in SBP, DBP, MBP, and HR was found in both the rhEPO and CRF rats. The rhEPO treatment in the CRF rats showed a trend to attenuate the BP values, and significantly (p < 0.05) corrected HR ( and ).
Table 3. Effects of rhEPO treatment on blood pressure, body and tissue weights, and peripheral catecholamine measures in a rat model of moderate CRF, at the final time (15 weeks)
Figure 1. Effects of rhEPO treatment on heart rate and left ventricle sympathetic nervous system activity in a rat model of moderate CRF, at the final time: heart rate (A) and left ventricle content in norepinephrine (B) and epinephrine (C). Results are means ± SEM (seven rats per group): ap < 0.05 and aaap < 0.001 versus the control group; bp < 0.05 versus the CRF group.
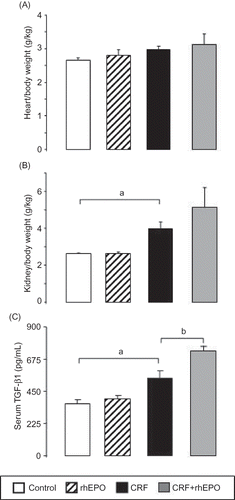
Concerning the proliferation/trophism measures, there was a trend for increased HW/BW in the CRF rats, together with a significant (p < 0.05) increment in KW/BW (which started with only half of the kidney) when compared with the control animals ( and , respectively). This profile was accompanied by a significant (p < 0.05) augment of serum TGF-β1 (). In the CKD + rhEPO group, both the heart and, in particular, the kidney showed a further trend to higher values when compared with the CRF animals without rhEPO treatment, together with a further significant increment in serum TGF-β1 levels ().
Figure 2. Effects of rhEPO treatment on trophism/proliferation markers: heart/body weight (A); kidney/body weight (B); and serum-transforming growth factor-1beta (C) in a rat model of moderate CRF, at the final time. Results are means ± SEM (seven rats per group): ap < 0.05 versus the control group; bp < 0.05 versus the CRF group.
Peripheral sympathetic nervous system activity
In the rhEPO rats, the NE (p < 0.001) and E (p < 0.05) plasma levels were significantly higher than those of the control group (). The CRF rats presented a statistically significant increase for NE in plasma (p < 0.05) and LV (p < 0.05), a reduction (p < 0.05) in platelets and a trend to diminution in adrenals ( and ). Similar profile was obtained for E, with a trend to higher values in plasma, a significant (p > 0.05) augment in the LV (), and reduction in platelets and adrenals (p > 0.05) (). rhEPO treatment in CRF rats promoted an almost complete prevention of the above-mentioned changes: there was a trend to correction of NE changes in plasma and a significant (p < 0.05) prevention of the LV contents increase observed in the CRF rats ( and ). The changes in E content were significantly (p < 0.05) prevented in plasma, adrenals, and LV ( and ).
Kidney gene expression of EPO, Casp9, and Vegf
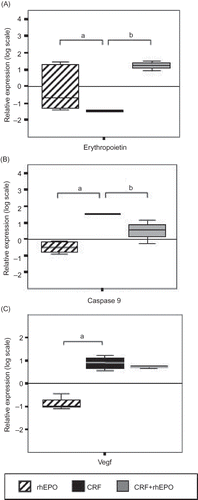
There was a significant (p < 0.05) downregulation of EPO gene expression in CRF rats, totally prevented (p < 0.05) by concomitant rhEPO treatment (). Evaluation of Casp9 gene expression gave evidence of a CRF-induced apoptosis, with a significant (p < 0.05) overexpression in the CRF group. rhEPO treatment in CRF + rhEPO rats significantly (p < 0.05) attenuated the increment of Casp9 (). The gene expression of Vegf gene was upregulated (p < 0.05) in the kidney tissue from CRF and CRF + rhEPO groups, with respect to the downregulation observed for the rhEPO group ().
Figure 3. Relative kidney gene expression quantification for erythropoietin (A); Caspase 9 (B); and Vegf (C) in rhEPO, CRF, and CRF + rhEPO groups comparatively to control group (zero line). Results are means ± SEM (seven rats per group): ap < 0.05 versus the control group; bp < 0.05 versus the CRF group.
DISCUSSION
Earlier rhEPO therapy in renal failure patients, moderate stages of CKD, might have a positive impact on renal and cardiovascular functions, because of its pleiotropic effects previously reported in heart failure.Citation13 Our model of surgical partial (3/4) nephrectomy, previously characterized by our groupCitation18, produces a moderate, but sustained, stage of CRF. Moreover, we observed a significant increase in serum urea and creatinine concentrations at 3 weeks after the surgery in the rats of the CRF group. During the following period, those values remained significantly high for CRF rats; however, in the animals of the CRF + rhEPO group, after 12 weeks of rhEPO treatment, creatinine and urea serum levels returned to control values, suggesting a slight recovery of renal function.
Anemia is a common complication associated with CKD patients that has been identified as an independent risk factor for progression of the disease, resulting in a significant morbidity and mortality in these patients.Citation5,Citation6 In our study, CRF rats showed a decrease in RBC count and HCT and Hb concentration, 3 weeks after nephrectomy, consistent with the development of anemia secondary to renal mass reduction. However, anemia is notoriously transitory, as all those parameters returned to the normal values at the final time (15 weeks). Moreover, we observed also that serum EPO levels at the end of treatments were similar for all groups, except for the rhEPO group that presented a significantly higher value, reflecting the exogenous treatment of rhEPO, associated to a normal renal function. However, in the kidney, the gene expression of EPO was reduced in the CRF rats and recovered in those animals with CRF and under rhEPO therapy; suggesting that rhEPO is able to recover renal tissue erythrocytosis impairment. The almost normal levels of serum EPO in the CRF rats suggest, therefore, the development of compensatory physiological mechanisms to overcome the reduced endogenous EPO synthesis by the nontotally functional kidney, which is in agreement with a recent study that has suggested the existence of nonrenal pathways.Citation22 We also found an increased kidney weight (hypertrophy), which is an even more relevant consideration that corresponds to only one-fourth of the whole tissue, together with augmented serum levels of TGF-β1 and kidney overexpression of Vegf, further suggesting an increase in renal tissue proliferation/hypertrophy, consistent with a compensated renal insufficiency. Serum levels of TGF-β1 are significantly higher in CRF rats treated with rhEPO, suggesting that rhEPO may contribute to increase remnant kidney hypertrophy. This observation suggests a probable mechanism contributing to relative renal recovery function associated with rhEPO treatment. Furthermore, the overexpression of Casp9 in the CRF rats was corrected with rhEPO treatment, further reinforcing the renoprotective role of rhEPO in the remnant kidney.
Besides the anemia, secondary to renal failure, CRF patients usually develop cardiac failure that further worsens renal disease. This triad of dysfunctions, already known as cardio-renal anemia syndrome, is responsible for serious disorders.Citation4–8 Hypertension is a well-established cause of cardiovascular disease, a common complication in CKD, and, therefore, an important risk factor for the progression of cardiovascular alterations and mortality of CKD patients.Citation23 Additionally, treatment with rhEPO has also been associated with hypertension.Citation24–26 Our results confirmed these conditions, with increased BP, together with tachycardia, peripheral sympathetic overactivity, and heart hypertrophy, in both the CRF and the rhEPO groups. However, the HR increase (tachycardia) in CRF rats was prevented in the group under rhEPO treatment, which might be due to a reduction of sympathetic overactivity, viewed by the prevention of NE and E increment in the left ventricle and in plasma. The contrary behavior found between the control rhEPO group, without nephrectomy (increased plasma NE and E contents), and the nephrectomized CRF + rhEPO group might be explained by the distinct requirements of the hormone in the two groups. Therefore, when needed, rhEPO promoted protection, contrasting with the eventually deleterious pro-sympathetic effects encountered in the group with anemic disorders and rhEPO requirement. These effects were similar to those found by our group in animals under regular exercise treatment and rhEPO therapy, in which there was no utility for rhEPO use, and the resulting effects were detrimental.Citation27 Sympathetic overactivity is a common feature observed in CKD patients,Citation28 which might result from the development of anemia and heart insufficiency, thus needing a mobilization of catecholamines to promote increased beating (tachycardia) to promote blood flux to peripheral tissues. This mechanism seems to be prevented when rhEPO is used in a moderate stage of CRF. It seems that rhEPO promotes heart hypertrophy, thus compensating the putative insufficiency that anemia could originate, alleviating the left ventricle from an additional effort, and thus preventing cardiac complications.
In conclusion, our data are consistent with a model of moderate, but sustained, degree of CRF, with development of moderate and corrected anemia, hypertension, and tachycardia. In this model, rhEPO treatment was able to improve renal function and has a pro-proliferative and anti-apoptotic action, together with a reduction of sympathetic overactivity and heart rate. As the pathophysiological modifications underlying CKD seems to be influenced by the lower endogenous EPO concentrations, this study suggests that rhEPO therapy might be earlier recommended in CKD patients (moderate stages) to efficiently correct not only the anemia but also the underlying deleterious mechanisms. This beneficial action seems to be because of a pleiotropic cardio-renal action, most probably attributed to its pro-proliferative, antioxidant, and anti-apoptotic properties that should be further tested in clinical studies with patients in earlier stages of CKD.
Declaration of interest: The authors report no conflict of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Deicher R, Hörl WH. Anaemia as a risk factor for the progression of chronic kidney disease. Curr Opin Nephrol Hypertens. 2003;12:139–143.
- Stevens LA, Levin A. Anaemia, cardiovascular disease and kidney disease: Integrating new knowledge in 2002. Curr Opin Nephrol Hypertens. 2003;12:133–138.
- Smrzova J, Balla J, Bárány P. Inflammation and resistance to erythropoiesis-stimulating agents – What do we know and what needs to be clarified? Nephrol Dial Transplant. 2005;20:2–7.
- Foley RN, Parfrey PS, Harnett JD, Kent GM, Murray DC, Barre PE. The impact of anaemia on cardiomyopathy morbidity and mortality in end-stage renal disease. Am J Kidney Dis. 1996;28:53–61.
- Locatelli F, Conte F, Marcelli D. The impact of hematocrit levels and erythropoietin treatment on overall and cardiovascular mortality and morbidity – The experience of the Lombardy Dialysis Registry. Nephrol Dial Transplant. 1998;13: 1642–1644.
- Silverberg D, Wexler D, Blum M, Wollman Y, Iaina A. The cardio-renal anaemia syndrome: Does it exist? Nephrol Dial Transplant. 2003;18:7–12.
- Palazzuoli A, Gallotta M, Iovine F, Nuti R, Silverberg DS. Anaemia in heart failure: A common interaction with renal insufficiency called the cardio-renal anaemia syndrome. Int J Clin Pract. 2008;62:281–286.
- Fluck R. Controversies in chronic kidney disease, anaemia and cardiovascular disease. Br J Hosp Med. 2008;69:580–586.
- McClellan WM, Jurkovitz C, Abramson J. The epidemiology and control of anaemia among pre-ESRD patients with chronic kidney disease. Eur J Clin Invest. 2005;35:58–65.
- Maiese K, Li F, Chong ZZ. New avenues of exploration for erythropoietin. JAMA 2005;293:90–95.
- Latini R, Brines M, Fiordaliso F. Do non-hemopoietic effects of erythropoietin play a beneficial role in heart failure? Heart Fail Rev. 2008;13:415–423.
- Bogoyevitch MA. An update on the cardiac effects of erythopoietin cardioprotection by erythropoietin and the lessons learnt from studies in neuroprotection. Cardiovasc Res. 2004;63:208–216.
- Manolis AS, Tzeis S, Triantafyllou K, Erythropoietin in heart failure and other cardiovascular diseases: Hematopoietic and pleiotropic effects. Curr Drug Targets Cardiovasc Haematol Disord. 2005;5:355–375.
- Shang Y, Wu Y, Yao S, Wang X, Feng D, Yang W. Protective effect of erythropoietin against ketamine-induced apoptosis in cultured rat cortical neurons: Involvement of PI3K/Akt and GSK-3 beta pathway. Apoptosis. 2007;12:2187–2195.
- Yang CW, Li C, Jung JY, Preconditioning with erythropoietin protects against subsequent ischemia-reperfusion injury in rat kidney. FASEB J. 2003;17:1754–1755.
- Abdelrahman M, Sharples EJ, McDonald MC, Erythropoietin attenuates the tissue injury associated with hemorrhagic shock and myocardial ischemia. Shock. 2004;22:63–69.
- Ma R, Xiong N, Huang C, Erythropoietin protects PC12 cells from beta-amyloid(25–35)-induced apoptosis via PI3K/Akt signalling pathway. Neuropharmacology. 2009;56: 1027–1034.
- Garrido P, Reis F, Costa E, Characterization of a rat model of moderate chronic renal failure – Focus on hematological, biochemical and cardio-renal profiles. Ren Fail. 2009; 31:833–842.
- Reis F, Rocha L, Ponte L, Effect of preventive and regressive isosorbide 5-mononitrate treatment on catecholamine levels in plasma, platelets, adrenals, left ventricle and aorta in cyclosporin A-induced hypertensive rats. Life Sci. 2005;77:2514–2528.
- Vandesompele J, De Preter K, Pattyn F, Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:R34.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25:402–408.
- Weidemann A, Johnson RS. Nonrenal regulation of EPO synthesis. Kidney Int. 2009;75:682–688.
- Fernandez-Fresnedo G, Rodrigo E, de Francisco AL, de Castro SS, Castañeda O, Arias M. Role of pulse pressure on cardiovascular risk in chronic kidney disease patients. J Am Soc Nephrol. 2006;17:S246–S249.
- Vaziri ND. Mechanism of erythropoietin-induced hypertension. Am J Kidney Dis. 1999;33:821–828.
- Smith KJ, Bleyer AJ, Little WC, Sane DC. The cardiovascular effects of erythropoietin. Cardiovasc Res. 2003;59:538–548.
- Jie KE, Verhaar MC, Cramer MJ, Erythropoietin and the cardiorenal syndrome: Cellular mechanisms on the cardiorenal connectors. Am J Physiol Renal Physiol. 2006;291:F933–F944.
- Piloto N, Teixeira HM, Teixeira-Lemos E, Erythropoietin promotes deleterious cardiovascular effects and live risk in a rat model of chronic sports doping. Cardiovasc Toxicol. 2009;9:201–210.
- Koomans HA, Blankestijn PJ, Joles JA. Sympathetic hyperactivity in chronic renal failure: A wake-up call. J Am Soc Nephrol. 2004;15:524–537.
