Abstract
Objective: Left ventricular hypertrophy (LVH) is the strongest predictor of cardiovascular mortality, the leading cause of death in hemodialysis (HD) patients. This study aims to identify the potential risk factors for LVH in HD patients. Methods: Exactly, 164 patients (84 men and 80 women) who had been on HD treatment for at least 6 months were enrolled. Clinical data were collected. Anthropometric measurements, biochemical analyses, and echocardiography were performed. The risk factors were determined by multivariate linear and logistic regression. Results: In all the patients, the prevalence of LVH was 66.5%. The patients with LVH had higher body mass index (BMI), systolic blood pressure (SBP), diastolic blood pressure, and lower single-pool kt/V (spKt/V) compared with those without LVH. Multivariate linear regression showed that BMI (β = 7.608, p = 0.014), SBP (β = 9.462, p = 0.001), and spKt/V (β = –14.226, p = 0.024) were independently correlated with left ventricular mass index (LVMI). Multivariate logistic regression showed the same results that BMI (β = 7.193, p = 0.032), SBP (β = 9.382, p = 0.02), and spKt/V (β = –12.535, p = 0.001) were independently correlated with LVH. Conclusions: In Chinese maintenance hemodialysis patients, BMI, single-pool Kt/V (spKt/V), and SBP were independently correlated with left ventricular mass index and were independent risk factors for LVH.
INTRODUCTION
In end-stage renal disease (ESRD), the prevalence of cardiac disease is very high.Citation1 It is the leading cause of death among maintenance hemodialysis (MHD) patients. Left ventricular hypertrophy (LVH) is a common complication that contributes substantially to high cardiovascular mortality and morbidity in ESRD.Citation2 The prevalence of LVH is also very high, ranging from 60% to 80%.Citation3,Citation4 It can occur very early in the progression of chronic kidney disease and may be present at the beginning of dialysis treatment.Citation5
LVH is currently considered the strongest predictor of cardiovascular and total mortality in the hemodialysis (HD) population. Up to now, there has been no consensus on the risk factors related to LVH. This study was conducted on MHD patients in China to identify potentially modifiable risk factors for better intervention in future.
PATIENTS AND METHODS
Patients
Briefly, 164 patients (84 men and 80 women) who had been on HD treatment for at least 6 months (median duration of dialysis treatment, 48 months; interquartile range, 24–78 months) were enrolled from Blood Purification Center, Zhongshan Hospital, Fudan University, Shanghai, China. These patients represented about 90% of the whole HD population in our center. The enrollment was completed within 1 month from 15 July to 15 August 2009. In our center, all HD patients were advised to have a high-protein diet (at least 1.2 g/kg/d, with mainly animal protein). The study was approved by the Ethical Committee, Zhongshan Hospital, Fudan University, and all the patients provided written informed consent.
Patients were treated thrice weekly with standard bicarbonate dialysate (Na+ 138.0 mmol/L, HCO3− 32.0 mmol/L, K+ 2.0 mmol/L, Ca2+ 1.25 mmol/L, Mg2+ 0.5 mmol/L) by low-flux HD using 1.4 m2 dialyzers with synthetic membranes (BLS514SD, Sorin Group Italia, Mirandola, Italy; Polyflux14L, Gambro Dialysatoren GmbH, Hechigen, Germany). The blood flow was 200–280 mL/min, and dialysate flow was 500 mL/min. Dry weight was targeted in every patient to achieve an edema-free state.
Blood Pressure Measurements
Blood pressure was estimated by averaging all predialysis blood pressure during the month before this study (12 measurements in total).
Anthropometric Measurements and Clinical Data Collection
Height and weight were measured while participants were barefoot and wearing light clothes only. The body mass index (BMI) was calculated as weight (kg) divided by height (m) squared. Demographic and clinical data were collected, including age, gender, smoking history, underlying kidney disease, dialysis duration, comorbidity, initial urine output at the onset of HD, and current urine output, on which urine reduction rate was determined.
Biochemical Measurements
Blood sampling was performed during a midweek nondialysis day 8–10 am after 30 min of quiet rest in semirecumbent position. Serum albumin, pre-albumin, hemoglobin, blood urea nitrogen, creatinine, calcium, phosphorus, lipids, homocysteine, ion, ferritin, and transferrin were measured using standard methods in the routine clinical laboratory. The concentrations of high-sensitivity C-reactive protein and β2-microglobulin were determined using immunoturbidimetry assay, and concentrations of intact parathyroid hormone (iPTH) were measured using electrochemiluminescence immunoassay.
Echocardiography
Transthoracic echocardiographic examinations were conducted using a Philips echocardiographic machine (Philips IE33, Eindhoven, the Netherlands) with a 3.5-MHz multiphase array probe by a single experienced cardiologist during a midweek nondialysis day, within 2 h after blood sampling. Measurements of the left ventricular internal dimension, interventricular septal thickness, and posterior wall thickness were made at end diastole according to the recommendations of Penn Convention. Left ventricular mass was calculated with the Devereux formula,Citation6 and left ventricular mass index (LVMI) was obtained by dividing left ventricular mass by height in meters raised to the power of 2.7. The left ventricular ejection fraction was determined by two-dimensional echocardiography.
Statistical Analysis
All data were expressed as mean ± SD or median (interquartile range) appropriately. To compare two groups of normal variables, independent samples t-test was used, whereas for skewed and categorical variables Mann–Whitney U and chi-square test were performed, respectively. One-way ANOVA and Kruskal–Wallis test were employed for analyzing multigroup of variables. Linear and logistic regressions were performed to identify the independent risk factors for LVMI and LVH. Covariates with p < 0.1 in the univariate regression were selected for multivariate regression analysis. Two-sided p < 0.05 was considered significant. All analyses were performed using SPSS version 17.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Patient Characteristics
One hundred and sixty-four HD patients (84 men and 80 women) participated in this study. The mean age was 57.0 ± 13.8 years. The median dialysis duration was 48 (24–78) months. The initial and current urine output were 21.1 ± 11.4 and 0 (0–5.05) mL/kg/24 h, respectively. The delivered dialysis dose measured by single-pool kt/V (spKt/V; second-generation Daugirdas equation) was 1.4 ± 0.3 or by urea reduction rate was 0.7 (0.6–0.7). Of the 164 patients, 52 had smoking history, 8 coronary heart disease, 20 type 2 diabetes mellitus, 20 cerebral infarction, and 6 cerebral hemorrhage. On echocardiographic examination, valvular diseases were found in nine patients (one moderate aortic regurgitation, two mild aortic regurgitation, one mild aortic stenosis, three mild mitral regurgitation, and two mild tricuspid regurgitation). Altogether 119 patients were on antihypertensive treatment (32 on monotherapy with calcium channel blocker, angiotensin-converting enzyme inhibitor, angiotensin-II receptor blocker, β-blocker or α-blocker, and 87 on double or triple therapy with various combinations of these drugs), whereas 123 patients were taking oral calcium salt, with calcitriol prescribed according to the serum iPTH level. Only 12 patients were taking statin lipid-lowering drugs. All patients received erythropoietin treatment, and intravenous iron was used when necessary. The underlying kidney diseases are listed in .
Table 1. The underlying kidney diseases of 164 MHD patients
The demographic, anthropometric, and clinical characteristics and biochemical parameters were given in . The average BMI was 22.0 ± 3.1 kg/m2, and patients with LVH had higher BMI than those without LVH (22.4 ± 3.4 vs. 21.2 ± 2.2 kg/m2, p = 0.047). In both groups, the average blood pressure was 136.6 ± 16.8/90.0 ± 9.8 mmHg. Patients with LVH had significantly higher systolic blood pressure (SBP) and diastolic blood pressure (DBP) compared with those without LVH (140.2 ± 14.6/83.5 ± 9.7 vs. 129.5 ± 18.5/80.0 ± 9.2 mmHg, p < 0.0001 and p = 0.004, respectively). No significant difference was found among the biochemical parameters. With regard to dialysis adequacy, we found that patients without LVH had higher spKt/V (1.5 ± 0.3 vs. 1.3 ± 0.3, p < 0.0001) and urea reduction rate [0.71 (0.66–0.77) vs. 0.66 (0.61–0.73), p < 0.001] compared with those with LVH, whereas there was no significant difference in initial and current urine output, urine reduction rate, smoking history, or dialysis duration between patients with and without LVH (p = 0.359, p = 0.729, p = 0.591, p = 0.386, and p = 0.17, respectively).
Table 2. The demographic, anthropometric, clinical characteristics, and biochemical parameters of 164 MHD patients
Prevalence of LVH
LVH was defined by a LVMI of >47 g/m2.7 in women or >50 g/m2.7 in men.Citation7 The height-based indexing was specifically chosen to minimize any potential distortion attributable to extracellular volume expansion. According to this criterion, the prevalence of LVH in our study group was 66.5%.
Influence of Cigarette Smoking and Underlying Kidney Disease on LVMI
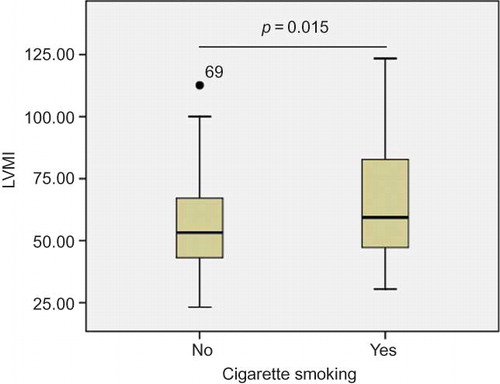
Of 164 MHD patients, 52 were smokers. The proportion of smokers to nonsmokers in the group with and without LVH were 37/72 and 15/40, respectively (p = 0.386, ). But in the whole group, smoking patients had higher LVMI than nonsmoking patients (63.5 ± 20.7 vs. 55.7 ± 18.2 g/m2.7, p = 0.015, ).
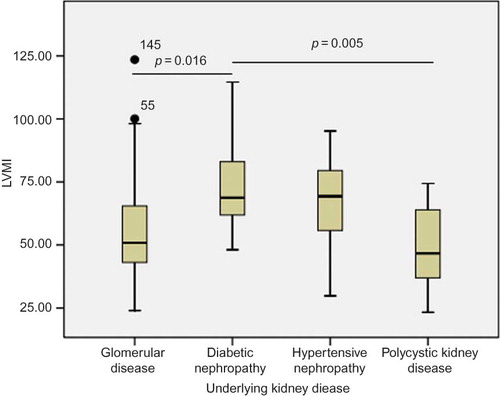
We investigated the relationship between LVMI and the top four underlying kidney diseases. The group of diabetic nephropathy had the highest LVMI of 73.7 ± 20.6 g/m2.7 compared with the group of hypertensive nephropathy (67.4 ± 18.9 g/m2.7), glomerular disease (56.2 ± 19.1 g/m2.7), and polycystic kidney disease (48.4 ± 20.0 g/m2.7) (p = 0.002). Post hoc analysis (Bonferroni method) showed significant difference in LVMI between the groups of diabetic nephropathy and glomerular disease (p = 0.016) and of diabetic nephropathy and polycystic kidney disease (p = 0.005, ). But the difference in LVMI between the group of hypertensive nephropathy and other three groups was not significant.
Linear Regression Model for LVMI and Related Factors
Univariate linear regression with LVMI as the dependent variable was performed. We found that BMI (β = 7.982, p = 0.042), SBP (β = 9.513, p < 0.0001), DBP (β = 6.698, p < 0.0001), and iPTH (β = 6.355, p = 0.043) were positively related to LVMI, whereas spKt/V (β = –16.709, p = 0.002) was inversely related to LVMI. There was also a tendency for dialysis duration (β = –6.99, p = 0.062) and serum iron (β = –0.444, p = 0.097) to be inversely related to LVMI. But age, gender, initial and current urine output, urine reduction rate, and other biochemical parameters were not correlated with LVMI. Then multivariate linear regression analysis was performed (backward stepwise method) with LVMI as dependent variable and BMI, SBP, DBP, spKt/V, iPTH, serum iron, dialysis duration, cigarette smoking, and underlying kidney disease as independent variables. The results showed that BMI (β = 7.608, p = 0.014), SBP (β = 9.462, p = 0.001), and spKt/V (β = –14.226, p = 0.024) were independently correlated with LVMI, whereas DBP (p = 0.936), iPTH (p = 0.603), serum iron (p = 0.907), dialysis duration (p = 0.883), cigarette smoking (p = 0.958), and underlying kidney disease (p = 0.462) had no significance ().
Table 3. Multivariate linear regression model with LVMI as the dependent variable in 164 MHD patients
Logistic Regression Model for LVH and Related Factors
First univariate logistic regression with LVH as dependent variable was performed. It was found that BMI (β = 8.141, p = 0.019), SBP (β = 10.043, p < 0.0001), DBP (β = 5.05, p = 0.006), and spKt/V (β = –14.12, p = 0.001) were significant, but iPTH (β = 2.628, p = 0.086) was marginally significant in contrast to the univariate linear regression. Based on the criterion of p < 0.1, we selected BMI, SBP, DBP, spKt/V, and iPTH as independent variables. It was also found that the results of multivariate logistic regression model (also backward stepwise method) were consistent with those of multivariate linear regression (). BMI (β = 7.193, p = 0.032) and SBP (β = 9.382, p = 0.02) were positively correlated with LVH, whereas spKt/V was inversely correlated with LVH (β = –12.535, p = 0.001). So it was suggested in this study that BMI, SBP, and spKt/V were the independent risk factors for LVH.
Table 4. Multivariate logistic regression model with LVH as the dependent variable in 164 MHD patients
DISCUSSION
Cardiovascular events are the main cause of death in MHD patients, whereas LVH is a common complication that contributes substantially to high cardiovascular morbidity and mortality in this population. Even in the predialysis population, the prevalence of LVH increases with progressive renal decline.Citation5 Based on the data from our center, we found that BMI, spKt/V, and SBP were the independent risk factors for LVH.
The association between BMI and mortality is controversial. Most researchers believed that there was a U-shaped or J-shaped relation between BMI and the risk of death in the general population.Citation8,Citation9 As for the HD population, greater BMI conferred a survival advantage.Citation10 Patients in the earlier stages of chronic kidney disease appear to represent the transition point between the general population and patients with kidney failure.Citation11
Concerning the relationship between BMI and LVH, the majority view was that higher BMI led to acquisition of LVMI and predicted LVH in general population including children and adults.Citation12,Citation13 In HD population, some studies showed that BMI was positively correlated with LVHCitation14 as in general population, whereas other studies gave different results.Citation15 In our study, the patients with LVH had higher BMI than those without LVH. Multivariate linear regression showed that BMI was positively correlated with LVMI, and such a positive correlation was elucidated by multivariate logistic regression.
Comparing the results of all previous studies about BMI and outcomes (mortality or LVH), we found there was no consensus especially in the HD population. This might be attributed to the confounding factors. First, the association was distorted owing to preexisting chronic disease and inadequate control for smoking status, because chronic illness and smoking were associated with decreased BMI and increased risk of the outcomes. Second, because of age-related changes in the amount and distribution of body fat, the impact of BMI on risk of mortality or LVH was dependent on the age and follow-up. Greater BMI was associated with higher mortality, but the relative risk declined with age.Citation16 Third, race was another confounding factor. Many studies found that with regard to the association between BMI and mortality, Asian people were an exception.Citation11,Citation17 The impact of BMI on LVH was likely to vary according to race. Notably, in our center, diabetic nephropathy accounts for only 7.9% of ESRD, which is lower than 30–50% in developed countries. Furthermore, the prevalence of disturbance of lipid metabolism is relatively low, which may be attributed to the relatively low prevalence of diabetic nephropathy. Last, the impact of BMI may be obscured by physical activity, alcohol use, dietary intake, and other pathologic process, including malnutrition, inflammation, and changes in vitamin D metabolism.
In 1990s, the United States Renal Data System; HEMO Study: the Hemodialysis Study (USRDS) reported that an approximate log-linear relationship of decreasing mortality with increased dose of dialysis existed within the range of spKt/V 0.8–1.4.Citation18 But the Hemodialysis (HEMO) Study gave a negative result, that is, neither the increased dose of dialysis (higher than that recommended by current US guidelines, i.e., spKt/V 1.2) nor the use of high-flux dialyzers improved outcomes.Citation19 As for the relationship between spKt/V and LVH, the limited data showed that spKt/V was not an independent risk factor for LVH.Citation20 However, when patients converted from conventional HD to daily HD with a longer time and so a higher spKt/V, LVMI decreased significantly.Citation21 In our study, spKt/V was negatively correlated with LVMI and an independent risk factor for LVH. This result could be explained by good control for volume overload, anemia, and hypertension with a higher spKt/V. Many studies indicated that the relative risk for morbidity and mortality in general and HD population was different concerning race and ethnicity. The association between spKt/V and LVH in Chinese people may be different from previously reported results. More studies are needed to elucidate this problem.
SBP but not DBP was a widely accepted risk factor for LVH in HD population,Citation22 and time-integrated SBP was an independent predictor of increase in LMVI in HD patients.Citation23 These results are consistent with our present findings. The increased cardiac afterload is suggested to be the major reason. But other potentially reversible factors such as anemia, volume overload, secondary hyperparathyroidism, uremic toxins, dose of dialysis, and malnutrition may also have an important role in the pathogenesis of LVH. All these confounding factors may have a part to play in the relationship between hypertension and LVH.
Although some studies indicated that active and long-term antihypertensive treatment could decrease LVMI,Citation24,Citation25 in this study, it was found that the use of antihypertensive medications including calcium channel blocker, angiotensin-converting enzyme inhibitor, angiotensin-II receptor blocker, β-blocker, and α-blocker had no significant relations with LVMI or LVH (data not listed). Multicenter, randomized, double-blind, controlled studies might give a definite answer in the future. Concerning the impact of SBP on LVH, the blood pressure control especially on SBP might be needed to alleviate progression of LVH.
CONCLUSIONS
From this cross-sectional study in Chinese MHD patients, we concluded that BMI, spKt/V, and SBP were independently correlated with LVMI and were the independent risk factors for LVH. The relationships between BMI, spKt/V, and LVH, as revealed in our study, are significantly different from the results of other countries, suggesting that race might be an important factor. Although they had a detrimental effect on LVMI, smoking and underlying kidney disease lost significance in multivariate regression. Studies of large sample sizes remain to be made to further elucidate this problem.
ACKNOWLEDGMENT
The authors are grateful to all the staff in Blood Purification Center, Zhongshan Hospital, Fudan University.
Declaration of interest: This study was supported by Shanghai Science and Technology Commission (08DZ1900602).
REFERENCES
- Cheung AK, Sarnak MJ, Yan G, Cardiac diseases in maintenance hemodialysis patients: Results of the HEMO study. Kidney Int. 2004;65:2380–2389.
- Lopez-Gomez JM, Verde E, Perez-Garcia R. Blood pressure, left ventricular hypertrophy and long-term prognosis in hemodialysis patients. Kidney Int Suppl. 1998;68:S92–S98.
- Parfrey PS, Foley RN, Harnett JD, Kent GM, Murray DC, Barre PE. Outcome and risk factors for left ventricular disorders in chronic uraemia. Nephrol Dial Transplant. 1996;11:1277–1285.
- Foley RN, Parfrey PS, Harnett JD, Clinical and echocardiographic disease in patients starting end-stage renal disease therapy. Kidney Int. 1995;47:186–192.
- Levin A, Singer J, Thompson CR, Ross H, Lewis M. Prevalent left ventricular hypertrophy in the predialysis population: Identifying opportunities for intervention. Am J Kidney Dis. 1996;27:347–354.
- Devereux RB, Reichek N. Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation. 1977;55:613–618.
- de Simone G, Daniels SR, Devereux RB, Left ventricular mass and body size in normotensive children and adults: Assessment of allometric relations and impact of overweight. J Am Coll Cardiol. 1992;20:1251–1260.
- Visscher TL, Seidell JC, Menotti A, Underweight and overweight in relation to mortality among men aged 40–59 and 50–69 years: The Seven Countries Study. Am J Epidemiol. 2000;151:660–666.
- Adams KF, Schatzkin A, Harris TB, Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. 2006;355:763–778.
- Kalantar-Zadeh K, Abbott KC, Salahudeen AK, Kilpatric RD, Horwich TB. Survival advantages of obesity in dialysis patients. Am J Clin Nutr. 2005;81:543–554.
- Madero M, Sarnak MJ, Wang X, Body mass index and mortality in CKD. Am J Kidney Dis. 2007;50:404–411.
- Stabouli S, Kotsis V, Rizos Z, Left ventricular mass in normotensive, prehypertensive and hypertensive children and adolescents. Pediatr Nephrol. 2009;24:1545–1551.
- Toprak A, Reddy J, Chen W, Srinivasan S, Berenson G. Relation of pulse pressure and arterial stiffness to concentric left ventricular hypertrophy in young men (from the Bogalusa Heart Study). Am J Cardiol. 2009;103:978–984.
- Vlahakos DV, Hahalis G, Vassilakos P, Marathias KP, Geroulanos S. Relationship between left ventricular hypertrophy and plasma renin activity in chronic hemodialysis patients. J Am Soc Nephrol. 1997;8:1764–1770.
- Ventura JE, Tavella N, Romero C, Petraglia A, Baez A, Munoz L. Aortic valve calcification is an independent factor of left ventricular hypertrophy in patients on maintenance hemodialysis. Nephrol Dial Transplant. 2002;17:1795–1801.
- Stevens J, Cai J, Pamuk ER, Williamson DF, Thun MJ, Wood JL. The effect of age on the association between body-mass index and mortality. N Engl J Med. 1998;338:1–7.
- Leavey SF, McCullough K, Hecking E, Goodkin D, Port FK, Young EW. Body mass index and mortality in ‘healthier’ as compared with ‘sicker’ hemodialysis patients: Results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol Dial Transplant. 2001;16:2386–2394.
- Held PJ, Port FK, Wolfe RA, The dose of hemodialysis and patients mortality. Kidney Int. 1996;50:550–556.
- Eknoyan G, Beck GJ, Cheung AK, Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med. 2002;347:2010–2019.
- Hsu HJ, Wu MS. Fibroblast growth factor 23: A possible cause of left ventricular hypertrophy in hemodialysis patients. Am J Med Sci. 2009;337:116–122.
- Maduell F, Navarro V, Torregrosa E, Change from three times a week on-line hemodiafiltration to short daily on-line hemodiafiltration. Kidney Int. 2003;64:305–313.
- Ishimitsu T, Nakano N, Sudo Y, Predictive significance of blood pressure values for the incidence of cardiovascular events in chronic hemodialysis patients. Hypertens Res. 2008;31:1703–1709.
- Foley RN, Curtis BM, Randell EW, Parfrey PS. Left ventricular hypertrophy in new hemodialysis patients without symptomatic cardiac disease. Clin J Am Soc Nephrol. 2010;5:805–813.
- Kutlay S, Dincer I, Sengul S, Nergizoglu G, Duman N, Erturk S. The long-term behavior and predictors of left ventricular hypertrophy in hemodialysis patients. Am J Kidney Dis. 2006;47:485–492.
- Matsumoto N, Ishimitsu T, Okamura A, Seta H, Takahashi M, Matsuoka H. Effects of imidapril on left ventricular mass in chronic hemodialysis patients. Hypertens Res. 2006;29:253–260.