Abstract
Herein we present one of the largest single-center reports of the response of hemodialysis patients to a two-vaccine hepatitis B virus vaccination protocol in a European dialysis population. A hepatitis B recombinant DNA vaccine, HBvaxPRO®, was given at a dose of 40 µg intramuscularly using a four-dose schedule at 0, 1, 2, and 12 months. Responses were (1) a titer >100 mIU/mL = patient immune, (2) a titer level 10–99 mIU/mL = give a booster dose and recheck level 2 months later, and (3) 0 £ 10 mIU/mL = repeat vaccination course using a different vaccine, Engerix-B®. We compared responder groups in terms of titer levels for each vaccine and variables including age, gender, serum albumin, parathyroid hormone (PTH), calcium, phosphate, hemoglobin, years on dialysis, and type of dialysis access. Of the 176 patients who received the first vaccine course, 71 patients achieved immunity, that is, 40% uptake for the first vaccine. Of the 105 who failed, 72 received the second vaccine with 46 responders, that is, 64% uptake for the second vaccine. Overall, 143 of the 176 patients who entered the vaccination program completed the protocol with 117 achieving immunity, representing an 82% success rate. The only variable overall to show significance in achieving seroconversion was serum albumin (p = 0.03). Using a two-vaccine protocol, hepatitis B vaccination response was high in our population of end-stage renal disease patients.
INTRODUCTION
Hepatitis B virus (HBV) infection can lead to serious complications including acute and chronic hepatitis, cirrhosis, hepatocellular carcinoma, and hepatic failure.Citation1 End-stage renal disease (ESRD) patients who undergo hemodialysis remain at high risk of contracting HBV infection. Once infected, about 60% of hemodialysis patients will become chronic carriers.Citation2 ESRD patients are recognized to have impaired immune responses including a suboptimal response to HBV vaccination.Citation3 Recent studies on B-cell and dendritic cell subpopulations in ESRD patients have provided evidence for possible mechanisms responsible for this reduced immune response to vaccination in ESRD patients.Citation4,Citation5 Dendritic cells act as antigen-presenting cells and have an essential function in the immune response. Agrawal et al.Citation4 have demonstrated a significantly lower circulating level of dendritic cells in ESRD patients, which is further reduced by the procedure of hemodialysis. Pahl et al.Citation5 have shown that ESRD patients on hemodialysis demonstrate significant reductions in B-cell subpopulations, contributing to their impaired humoral response to vaccination.
Vaccination leads to effective seroprotection in only 50–80% of ESRD patients and fewer develop protective antibodies against HBV.Citation6 Following a case of reactivation of HBV infection in a patient undergoing hemodialysis in our institution, a program of HBV serological testing and a two-vaccine protocol of HBV vaccination was instituted for all those at potential risk.Citation7 Herein, we report on its outcome and the potential factors influencing seroconversion.
MATERIALS AND METHODS
This was a prospective study initiated in response to a case of reactivation of HBV infection in a patient on hemodialysis in our unit.Citation7 Our unit was a hepatitis B-free unit when this event occurred. The incident highlighted that at the time there were a variety of strategies for HBV vaccination and testing in place and the tracking of the vaccination schedule was incomplete for many patients. Patients were entered into the study when there was no clear documentation of prior HBV vaccination, testing, booster administration, and adequate titre levels reflecting vaccine immunity. No patient had undergone a two-vaccine protocol of HBV vaccination in the past.
The two-vaccine protocol of the hepatitis B vaccination program was conducted over a 3-year period, 2005–2008. We reviewed all ESRD patients suitable for vaccination meeting the criteria for hepatitis B vaccination, that is, patients on chronic hemodialysis and not already immune (HBsAg and Anti-HBc negative). The exclusion criteria were a previous allergic reaction to the vaccine or an allergy to yeast, being hepatitis B core positive, or prior vaccine immunity with clear documentation of adequate titers post vaccination. A dedicated hepatitis B vaccination database was created and maintained by the renal virology nurse specialists in the renal unit. The data entered included all hemodialysis patient identifiers, date of vaccination dose administration, date of titer levels due, administered and subsequent results, date of booster dose due, administered and subsequent results. The virology nurse specialists ensured that patients received vaccination doses and booster doses and that titer levels were taken on the correct dates in accordance with the vaccination schedule. Patient information and virology results were obtained from hospital medical records and the renal unit database (Clinical Vision©, Clinical Computing, Cincinnati, OH, USA).
In the first year of the study, 135 patients in our unit were considered for vaccination. Of these, 97 were vaccinated and 38 were not vaccinated for the following reasons: 17 had prior documented vaccine immunity, 11 were hepatitis B core positive, 8 died mid vaccination, 1 had an allergy, and 1 patient refused. A further 79 patients were recruited over the remaining study period.
In total, 176 patients were vaccinated. These included all prevalent patients on chronic hemodialysis at the start of the study eligible for vaccination and all new patients commencing dialysis during the study period.
The initial vaccine used was a hepatitis B recombinant DNA vaccine, HBvaxPRO® (Sanofi Pasteur MSD, Lyon, France). The protocol for vaccination is described as follows: HBvaxPRO® was given at a dose of 40 µg intramuscularly into the deltoid muscle, using a four-dose schedule at 0, 1, 2, and 12 months. Post vaccination, an anti-HBs titer was taken at 2 months post the last dose. Responses were (1) a titer >100 mIU/mL = patient immune, (2) a titer level 10–99 mIU/mL = give a booster dose and recheck level 2 months later, and (3) 0 £ 10 mIU/mL = repeat the vaccination course using a different vaccine. For nonresponders, Engerix-B® (GlaxoSmithKline Biologicals, Rixensart, Belgium) was given as the second vaccine. For the vaccine responders and nonresponders, we compared age, gender, serum albumin, parathyroid hormone (PTH), calcium, phosphate, hemoglobin, years on dialysis, and type of dialysis access.
Statistical Analysis
Association of titer level to various demographic variables was conducted using Kruskal–Wallis rank tests for continuous variables and Pearson chi-squared test for categorical variables. A logistic regression model to determine clinical and demographic variables associated with response using the combined strategy of vaccination was also conducted. Statistical software used was Stata™ (version 10, College Station, TX, USA). A p-value of <0.05 was considered significant.
RESULTS
Demographic characteristics of the vaccinated group included 111 (63%) male and 65 (37%) female patients. The average age of males was 59 years and females 62 years; 96% of the patients were Caucasian. Details of immune response along with demographic details are presented in .
Table 1. Demographic analysis of factors affecting immune response of HBvaxPRO®
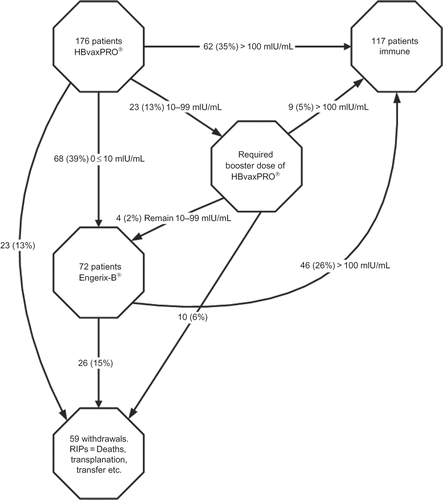
demonstrates the flow of patients through the vaccination program. It shows 71 patients in total achieving immunity following the first vaccine course, representing a 40% uptake. For the 72 patients administered the second vaccine Engerix-B®, 46 responded, that is, 64% uptake of the second vaccine. The remaining 33 patients, who failed the initial vaccine, either refused the second vaccine or were censored due to transplantation or death (there were five deaths recorded).
Overall, 143 of the 176 patients who entered the vaccination program completed the two-vaccine protocol with 117 achieving immunity, an 82% success rate. We compared responder groups in terms of overall response for the two vaccines. Results of a multifactorial model detailing risk factors in seroconversion are presented in . Only low albumin level is a predictor of poor uptake. Incidentally, no patient developed hepatitis B during the study period.
Table 2. Factors influencing seroconversion in ESRD patients taking either HBvaxPRO® or Engerix-B®
DISCUSSION
To our knowledge, this is one of the largest prospective studies of the response of hemodialysis patients to a two-vaccine protocol of HBV vaccination in a European single-center dialysis population. The Centers for Disease Control and Prevention suggest that vaccine uptake should aim for between 67% and 86% in hemodialysis patients. In our patient population, there was only 40% uptake of the first vaccine but 64% uptake of the second vaccine. Overall, 143 of the 176 patients who entered the vaccination program completed the protocol with 117 achieving immunity, representing an overall 82% success rate. These findings strongly support a vaccination protocol using two-vaccine courses. However, in the absence of a control group, we can only support the use of two courses of a vaccine, as giving a second course of HBvaxPRO® may have been as effective as switching to Engerix-B®.
It has been suggested that response rates to revaccination of nonresponders can vary from 40% to 50%.Citation8 Our response rates in nonresponders to revaccination were well above this at 64%. This high response to the second vaccination in the nonresponders was of interest. Studies have shown that healthy responders to HBV vaccination develop an immune memory response and maintain it, even after their anti-HBs antibody titer diminishes below the accepted level.Citation9 In our study, nonresponders to the first vaccine course did not demonstrate adequate titer levels to qualify them as vaccine immune. However, it is possible that they attained some immune memory from the first vaccine, which facilitated an improved response to the second vaccine. Overall they also received a higher antigen concentration load with the two-vaccine protocol, which may have influenced the higher response rate seen with the second vaccine.
In our patients, using the first vaccine, serum albumin was a significant factor in attaining seroconversion, similar to previous reports.Citation6 There is a known association between older age and increased risk of nonresponse to HBV vaccination among dialysis patients.Citation10,Citation11 Certainly the younger age profile in our population, the average age of males was 59 years and females 62 years, contributed to the good response rates. Studies have also suggested that years on dialysis and male gender gave a better response.Citation11 However, in our study, we did not find that years on dialysis or male gender were significant variables.
During the vaccination program, two patients received plasma exchange treatment as part of a desensitization program pretransplantation. Their response to vaccination was poor. In the circumstances, vigilance when vaccinating patients who are receiving other treatments, which could interfere with their immune response, should be closely evaluated. These patients were excluded from the final analysis.
This vaccination program was initiated in response to a case of reactivation of HBV infection and so it was necessary to include all patients unless there was absolute evidence of HBV vaccine immunity. A limitation of the study is that some patients included may have received partial vaccination in the past and had developed possible immune memory. However, there were only 17 patients who had clear documented evidence of prior vaccination with adequate titers reflecting vaccine immunity and these were excluded. Also no patient enrolled in the study had undergone a two-vaccine protocol of HBV vaccination in the past.
There are now several other approaches to improve the outcomes and responses in ESRD patients to vaccination. The adjuvant HBV vaccine (Fendrix™, GlaxoSmithKline Biologicals, Rixensart, Belgium) contains as active substance 20 µg recombinant hepatitius B surface antigen and the novel adjuvant system composed of aluminum salt and 3-O-desacyl-4′-monophosphoryl lipid A (AS04).This HBV-AS04 vaccine appears to have a good safety profile and has elicited earlier antibody response and higher antibody titers in pre-hemodialysis and hemodialysis patients as compared with four double doses of standard HBV vaccine.Citation12 Some investigators have suggested that the administration of tetanus toxoid 3 days before HBV vaccination, especially in diabetic dialysis patients, may enhance the immune response.Citation13
In conclusion, hepatitis B vaccination response in our population of ESRD patients was very successful at 82%. In our cohort of patients, serum albumin (p = 0.03) was an important factor in attaining seroconversion. Our findings suggest that successful hepatitis B vaccination can be achieved in ESRD populations.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Wong PN, Fung TT, Mak SK, Hepatitis B virus infection in dialysis patients. J Gastroenterol Hepatol. 2005;20 (11): 1641–1651.
- Tong NK, Beran J, Kee SA, Immunogenicity and safety of an adjuvanted hepatitis B vaccine in pre-hemodialysis and hemodialysis patients. Kidney Int. 2005;68(5):2298–2303.
- Yoon JW, Gollapudi S, Pahl MV, Vaziri ND. Naïve and central memory T-cell lymphopenia in end-stage renal disease. Kidney Int. 2006;70(2):371–376.
- Agrawal S, Gollapudi P, Elahimehr R, Pahl MV, Vaziri ND. Effects of end-stage renal disease and hemodialysis on dendritic cell subsets and basal and LPS-stimulated cytokine production. Nephrol Dial Transplant. 2010;25(3):737–746.
- Pahl MV, Gollapudi S, Sepassi L, Gollapudi P, Elahimehr R, Vaziri ND. Effect of end-stage renal disease on B-lymphocyte subpopulations, IL-7, BAFF and BAFF receptor expression. Nephrol Dial Transplant. 2010;25(1):205–212.
- Chin AI. Hepatitis B virus vaccine response in hemodialysis baseline patient characteristics. Hemodial Int. 2003;7:296–303.
- Thornton L, Fitzpatrick F, De La Harpe D, Hepatitis B reactivation in an Irish dialysis unit, 2005. Euro Surveill. 2007;12(4):E7–E8.
- Centres for Disease Control and Prevention. Recommendations for preventing transmission of infections among chronic hemodialysis patients. MMWR Recomm Rep. 2001;50(RR-5):1–43.
- Dentico P, Crovari P, Lai PL, Anamnestic response to administration of purified non-adsorbed hepatitis B surface antigen in healthy responders to hepatitis B vaccine with long-term non-protective antibody titers. Vaccine. 2002;20(31–32):3725–3730.
- Fabrizi F, Martin P, Dixit V, Bunnapradist S, Dulai G. Meta-analysis: The effect of age on immunological response to hepatitis B vaccine in end-stage renal disease. Aliment Pharmacol Ther. 2004;20(10):1053–1062.
- Lacson E, Teng M, Ong J, Vienneau L, Ofsthun N, Lazarus JM. Antibody response to Engerix-B and Recombivax-HB hepatitis B vaccination in end-stage renal disease. Hemodial Int. 2005;9(4):367–375.
- Beran J. Safety and immunogenicity of a new hepatitis B vaccine for the protection of patients with renal insufficiency including pre-hemodialysis and hemodialysis patients. Expert Opin Biol Ther. 2008;8(2):235–247 (review).
- Ocak S, Eskiocak AF. The evaluation of immune responses to hepatitis B vaccination in diabetic and non-diabetic hemodialysis patients and the use of tetanus toxoid. Nephrology (Carlton). 2008;13(6):487–491.