Abstract
Aim: To investigate the effects of lentivirus-mediated shRNA targeting collagen type I on the mesangial cells of rats and the feasibility of lentivirus-mediated shRNA delivery through renal parenchyma injection. Methods: Anti-collagen type I shRNA lentiviral vector was constructed, and rat mesangial cells were transfected with transfection enhancer (control group), blank lentiviral vectors (pSC-GFP group), and pSC-GFP/Col I lentiviral vectors (pSC-GFP/Col I group). Transfection efficiency and cell cycle were determined by flow cytometry. RT-PCR and Western blot were performed to detect the mRNA and protein expressions of Col I. Cell proliferation was evaluated by 3-(4,5)-dimethylthiahiazo-3, 5-di-phenytetrazolium-romide (MTT) assay and direct counting, and apoptosis was detected using AnnexinV/PE staining. The feasibility of renal parenchyma injection of lentiviral vectors was assessed. Results: The transfection efficiency was 75.42%. The expressions of collagen type I in pSC-GFP/Col I group was markedly decreased when compared with the other two groups. PSC-GFP/Col I group was higher than pSC-GFP group in the inhibition efficiency of mesangial cell after transfection. Results revealed that pSC-GFP/Col I transfection induced apoptosis to a certain extent. The proportion of cells in G2/M phase in pSC-GFP/Col I group and pSC-GFP group was higher than that in control group after of transfection. Moreover, cells arrested in S phase were markedly increased. Our results also revealed renal injection of lentivirus-mediated shRNA was feasible. Conclusion: Lentivirus-mediated shRNA targeting collagen type I could stably and efficiently transfect rat mesangial cells and significantly suppressed collagen type I expressions with acceptable safety. Renal injection of Col I lentivirus-mediated shRNA was also feasible.
INTRODUCTION
Renal fibrosis is a pathological feature of end-stage renal disease which is usually derived from various chronic kidney diseases progressively. Excessive aggregation of collagens is the main cause of renal fibrosis. Among the collagens, collagen type I is closely related to renal fibrosis and plays important role in the occurrence and development of renal fibrosis.Citation1 Under the regulation of pathological injury factors and some cytokines, collagen type I is over-expressed and extensively aggregates in the glomeruli and interstitium, involving in the pathological progress of renal injury (immune and inflammation) and leading to the occurrence and development of renal fibrosis.Citation2,Citation3 Therefore, strategies aiming to inhibit or downregulate collagen type I expression may be beneficial for the improvement of renal fibrosis.
RNA interference (RNAi) technique has been thought as an effective, specific, simple, and feasible tool in gene therapy due to its specificity, continuity, and sensitivity.Citation4–6 Currently, RNAi technique has been applied in some fields. In medicine, RNAi technique is used in the treatment of cancers, viral infection, and genetic diseases achieving remarkable achievements.Citation7,Citation8 RNAi technique has been a hot topic in applied clinical studies. One of the challenges to RNAi technique is the difficulty to control the specificity of RNAi in target organs, and the way in which shRNA was delivered is a bottleneck problem of RNAi, which limits its wide application. In addition, some researchers concern the side effects of shRNA lentiviral vectors on cells or on the whole body. To date, the effects of lentiviral vectors on the growth behavior of mesangial cells are still unknown.
In the present study, shRNA plasmid vectors were constructed, and lentiviral vectors carrying anti-collagen type I shRNA were prepared. The suppressive effect of collagen type I shRNA lentiviral vectors on the collagen type I expression in mesangial cells was detected and the transfection efficiency and interference efficiency were determined, which may be helpful for analysis of the efficacy and safety of shRNA lentiviral vectors as a drug for gene therapy. Based on these results, the feasibility of renal injection of shRNA lentiviral vectors was also investigated to find a novel way in which the progression of renal fibrosis can be delayed.
MATERIALS AND METHODS
Animals
Healthy male Sprague-Dawley rats weighing about 150 g were purchased from the experimental animal center. The serial number of animal quality certificate is SCXK-2007-004 and that of animal use license is SYXK2007-014.
Reagents and Equipment
Mesangial cells were stored in our department. Trizol (Invitrogen , Carlsbad, USA), D2000 DNA Marker (Tiangen , Beijing, PR China), Annexin V/PE kit (Bender Medsystems, Vienna, Austria), 7-AAD (BD Pharmingen , New Jersey, USA), collagen type I shRNA lentiviral vectors (Shanghai Genechem Co., Ltd ., Shanghai, PR China), mouse anti-rat IgG/Col I primary antibodies (Santa Cruz , Santa Cruz, USA), and rabbit anti-mouse IgG conjugated to horseradish peroxidase (Zhongshan Goldenbridge Biotech Co., Ltd ., Beijing, PR China) were used in our study. In addition, for Col I, the forward primer was 5′-GGTTTGGAGAGAGCATGACC-3′ and reverse primer was 5′-TTTGGGGAAATTGAGTTTGG-3′. For GAPDH, the forward primer was 5′-GGTGCTGAGTATGTCGTGGAGT-3′ and reverse primer was 5′-ACAGTCTTCTGAGTGGCAGTGAT-3′. The anticipated size of amplified Col I and GAPDH was 513 bp and 292 bp, respectively. The primers were synthesized in Shanghai Sangon Biotech Co., Ltd (Shanghai, PR China).
Construction of Col I shRNA Plasmids and Lentiviral Vectors
Based on the target sequence GCAACCTGGATGCCATCAA,Citation8 the corresponding primers were designed. Double-strand DNA oligonucleotides carrying interference sequences were synthesized by Shanghai Genechem Co., Ltd. These oligonucleotides have sticky ends of restriction recognition sites and directly connected to RNAi vectors which had undergone restriction digestion. The gene sequence of plasmid vector was finally confirmed as CCGGGCAACCTGGATGCCATCAATTCAAGA GATTGATGGCATCCAGGTTGCTTTTTG. The lentiviral vector system consisted of pGC-LV/pGC-Col I vector, pHelper1.0 vector, and pHelper2.0 vector. pGC-LV vector was blank vector carrying green fluorescent protein, and pGC-Col I was vector carrying green fluorescent protein and Col I shRNA. Then, endotoxin-free vectors with high purity were obtained and used to cotransfect 293T cells. Forty-eight hours later, the supernatants rich in lentiviral particles were collected followed by concentration. Subsequently, the lentiviral preparations were set as 108 TU/mL in 293T cells.
Cell Culture and Determination of Optimal Transfection Titer
Rat mesangial cells were maintained in DMEM medium containing 10% calf serum at 37°C in a humidified air with 5% CO2. After 2 days of culture, the cell density was adjusted to 2 × 104/mL and 500 µL of cell suspension were independently added into 4 wells (24-well plate) followed by incubation for 24 h. Then, transfection enhancer, 106 TU/mL, 107 TU/mL, or 108 TU/mL Col I shRNA lentiviral vectors were independently supplemented into each well followed by incubation for 96 h. Digestion was performed and single cell suspension was prepared. The amount of green fluorescent protein was determined by flow cytometry. Finally, the optimal transfection titer was determined based on the transfection efficiency and cytotoxicity of lentiviral vectors.
Detection of Transfection Efficiency of Col I shRNA Lentiviral Vectors
Cells were divided into three groups. Cells in control group were treated with transfection enhancer, those in pSC-GFP group with blank vector and transfection enhancer, and those in pSC-GFP/Col I group with Col I shRNA lentiviral vectors and transfection enhancer. Six hours later, cell density was adjusted to 5 × 104/mL with fresh medium and cells were plated. Ninety-six hours later, transfection efficiency was determined by flow cytometry.
Detection of Interference Efficiency of Col I shRNA Lentiviral Vectors
Interference efficiency at the transcription level
Rat mesangial cell (RMC) cells were seeded in a 24-well plate at a density of 2 × 104 cells/mL (500 µL/well) followed by incubation for 24 h. Then, these cells were assigned into three groups and transfection enhancer, blank lentiviral vectors, and Col I shRNA lentiviral vectors were added to control group, pSC-GFP group, and pSC-Col I group, respectively. After 2 days of culture, total RNA was extracted with Trizol and RNA quality was detected by 1.2% agarose gel electrophoresis. Reverse transcription was performed according to manufacturer's instructions (TaKaRa , Dalian, PR China). A total of 35 cycles of PCR were conducted and 5 µL of amplified products were loaded for agarose gel electrophoresis for 15 min followed by visualization and analysis of images.
Interference efficiency at the translation level
After 20 days of culture, medium was removed and cells were harvested. Total proteins were extracted and protein concentration was determined with a BCA kit. Then, 20 µg of proteins were separated by 10% SDS-PAGE and transferred onto PVDF membranes, which were blocked with 1% non-fat milk in Tris-Buffered Saline Tween (TBST) for 1 h. These membranes were incubated with mouse anti-rat primary antibody (1:1000) at 4°C overnight, and thereafter with rabbit anti-mouse IgG conjugated to horseradish peroxidase (1:5000) at room temperature for 1 h. Bands were visualized by enhanced chemiluminescence.
Detection of Cell Proliferation by MTT Assay
Cells were divided into three groups and experiment was performed in triplicates. After transfection, RMC cells (5 × 104/mL) were seeded in a 96-well plate (100 µL/well) followed by incubation for 24 h, 48 h, and 72 h. Then, 3-(4,5)-dimethylthiahiazo-3,5-di-phenytetrazolium-romide (MTT) was supplemented followed by incubation at 37°C in a humidified air with 5% CO2 for 4 h. Subsequently, 10% SDS was added and incubation was performed for 24 h. Opacity density (OD) was determined at 570 nm with a microplate reader. Experiment was conducted three times:
Detection of Proliferation by Direct Counting
Cell counting was performed in triplicates. After transfection, RMC cells were seeded into a 6-well plate at a density of 5 × 104/mL followed by incubation for 24 h, 48 h, and 72 h. Then, single cell suspension was prepared and cell counting was performed with a cell-counting slide.
Detection of Apoptosis by Annexin V/PE Staining
After 10 days of transfection, RMC cells were seeded into a 6-well plate followed by incubation for 24 h. Then, digestion and centrifugation were performed. Cells were harvested and resuspended in 100 µL of Annexin V-binding buffer and 5 µL of Annexin V/PE followed by incubation in dark at room temperature for 15 min. Then, 10 µL of 7-AAD (1 mg/mL) were added followed by incubation in dark for 5 min. Subsequently, 400 µL of PBS were supplemented and apoptosis was determined by flow cytometry.
Detection of Cell Cycle by Flow Cytometry
After transfection, RMC cells were seeded into a 6-well plate followed by incubation for 24 h, 48 h, and 72 h. Digestion and centrifugation were performed, and cells were resuspended in 0.3 mL of PBS containing 5% calf serum. Then, 0.7 mL of absolute ethanol was added to cell suspension followed by fixation for more than 24 h at –20°C. Subsequently, 200 µL of RNase A (1 mg/mL) were added followed by incubation at 37°C for 0.5 h. Cells were treated with 400 µL of PI solution (100 µg/mL) for 20 min and apoptosis was detected with a flow cytometer. Experiment was performed three times.
Renal Injection of Lentiviral Vectors
Ten healthy male Sprague-Dawley rats (specific pathogen free) weighing 185 ± 35 g were fasted for 12 h. Su-Mian-Xin II and ketamine were mixed at a ratio of 3:4 (v/v). Animals were intraperitoneally anesthetized with the mixture (0.7 mL/kg). Then, rats were fixed on board and the waist was elevated a little bit. An incision was made in the left lumbar back and psoas muscle was separated. The retroperitoneum was opened and left kidney exposed. Then, 5 × 107 TU Col I shRNA lentiviral vectors were injected into the lower pole of the left kidney at several sites. Retroperitoneum and skin were sutured. Ten days later, animals were sacrificed by cervical dislocation and abdominal cavity was opened. The lower pole of the left kidney and liver tissues at the hilum were obtained and kept in liquid nitrogen precooled isopentane for 2 min. Then, tissues were cut into 7 µm sections at –20°C followed by observation under inverted fluorescence microscope.
Statistical Analysis
Statistical analysis was performed with SPSS 11.0. Quantitative data were expressed as mean ± standard deviation. Comparisons between multiple groups were conducted with one-way ANOVA, and those between two groups with SNK test. A value of p < 0.05 was considered statistically significant.
RESULTS
Screening Optimal Transfection Titer
pSC-GFP/Col I lentiviral vectors can express GFP which was detected through flow cytometry and transfection efficiency was determined. Results indicated the transfection efficiency of 106 TU/mL, 107 TU/mL, and 108 TU/mL lentiviral vectors were 11.14%, 85.87%, and 94.91%, respectively. Furthermore, massive cell death was observed after transfection with 108 TU/mL lentiviral vectors. Therefore, the optimal transfection titer was determined as 107 TU/mL.
Transfection Efficiency of Lentiviral Vectors in Mesangial Cells
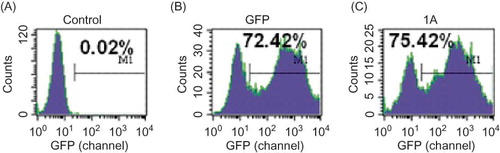
After transfection for 96 h, inverted fluorescence microscopy showed the mesangial cells with green fluorescence were those successfully transfected with lentiviral vectors. Flow cytometry indicated the transfection efficiency was 0.02% in control group, 72.42% in pSC-GFP group, and 75.42% in pSC-GFP/Col I group ().
Figure 1. Transfection efficiency in different groups at the titer of 107 TU/mL: (A) control group; (B) pSC-GFP group; (C) pSC-GFP/Col I group. The unit for the values in x-axis is channel. The transfection efficiency was 0.02% in control group, and that in pSC-GFP group and pSC-GFP/Col I group was similar. These findings suggested Col I shRNA did not affect the transfection.
Interference Efficiency of Lentivirus-Mediated Col I shRNA Interference in RMC
mRNA expression of Col I
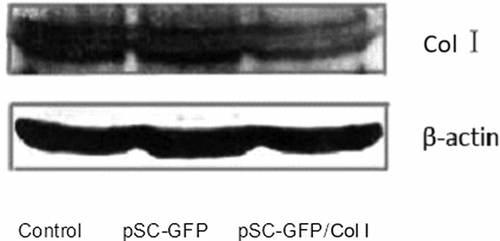
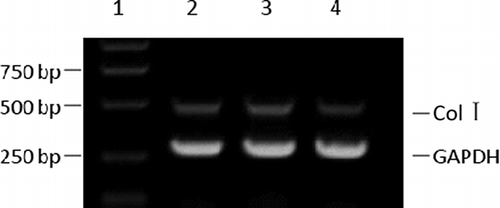
RT-PCR displayed a band at 513 bp which was identified as Col I α1. The OD was similar between control group and pSC-GFP group, but markedly decreased in pSC-GFP/Col I group. In addition, the band of GAPDH was also found in three groups ().
Figure 2. mRNA expression of Col I in mesangial cells (RT-PCR). 1, marker; 2, blank; 3, pSC-GFP; 4, pSC-GFP/Col I.
Images were analyzed by Scion Image analysis system and results showed the mRNA expression in pSC-GFP/Col I was significantly decreased when compared with control group (p < 0.05). This finding suggested transfection with pSC-GFP/Col I successfully and specifically inhibited the Col I expression with the inhibition rate of 32.76% ().
Table 1. OD in different groups (RT-PCR)
Protein expression of Col I
Results in Western blot were similar to those in RT-PCR (). There was no marked difference in protein expression of Col I between control group and pSC-GFP group, but the Col I expression in pSC-GFP/Col I group was remarkably decreased when compared with the remaining two groups (p < 0.05).
MTT Assay
MTT assay showed the OD in pSC-GFP/Col I group and pSC-GFP group was significantly lower than that in control group (p < 0.05) at all time points. Furthermore, the OD in pSC-GFP/Col I group was markedly lower than that in pSC-GFP group (p < 0.05). The inhibition rate was 22.52% in control group, 28.57% in pSC-GFP group, and 33.33% in pSC-GFP/Col I group ().
Table 2. OD in the detection of cell proliferation by MTT assay
Growth Curves Determined by Direct Cell Counting
Results indicated the growth of mesangial cells in control group was in an exponential manner, but that in pSC-GFP group and pSC-GFP/Col I group was markedly suppressed (p < 0.05). The suppressed growth was relatively stable and not changed significantly over time. Furthermore, the suppressed growth in pSC-GFP/Col I group was more obvious than that in pSC-GFP group (p < 0.05).
Cell Apoptosis
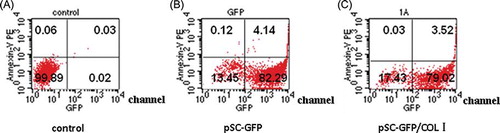
As shown in , the apoptotic rate was 0.09% in control group, 4.26% in pSC-GFP group, and 3.55% in pSC-GFP/Col I group.
Figure 4. Apoptosis through Annexin V/PE staining: (A) control group; (B) pSC-GFP group; (C) pSC-GFP/Col I group. The threshold limit value was set at 102 channel. The sum of proportions of cells in the upper left and right quadrants was the proportion of apoptotic cells. The proportion of apoptotic cells in pSC-GFP group and pSC-GFP/Col I group was markedly higher than that in control group.
Cell Cycle
The proportion of cells in G2/M phase in pSC-GFP/Col I group and pSC-GFP group was significantly higher than that in control group (p < 0.05) at 24 h, 48 h, and 72 h. After 72 h of transfection, the proportion of cells in S phase in pSC-GFP/Col I group was profoundly higher than that in control group and pSC-GFP group ().
Table 3. Proportion of cells in different phases after 24 h, 48 h, and 72 h of transfection
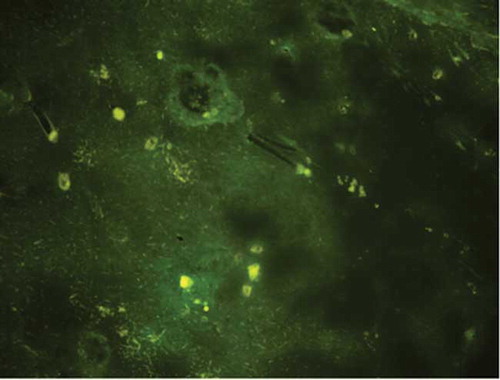
Expression of Green Fluorescent Protein Under Inverted Fluorescence Microscope After Renal Injection
After renal injection, the left kidney and liver tissues at the hilum were obtained for inverted fluorescence microscopy. Expression of green fluorescent protein was not observed in the liver but found around the glomerulus of renal cortex at injection sites (). We also observed the lower pole of left kidney structure (multi-injection area) under optical microscope. Kidney structure was normal by using HE staining.
Figure 5. Representative photograph of the kidney after renal injection from inverted fluorescence microscopy (200×) Lentivirus-mediated Col shRNA delivery was performed through injection into renal cortex and green fluorescent protein was determined under an inverted fluorescence microscope which reflects the feasibility of lentivirus-mediated shRNA delivery through renal parenchyma injection.
DISCUSSION
RNAi can efficiently and specifically downregulate target gene expression and has broad application prospects in gene therapy and studies of gene function.Citation9 Currently, lentiviral vectors have become a potential drug for gene therapy.Citation10 Apart from the therapeutic effects, the safety of RNAi is also a concern of numerous researchers. In the present study, lentiviral vectors of different titers were used to transfect rat mesangial cells aiming to find an optimal transfection titer. Our results showed the transfection efficiency increased in a titer-dependent manner. The higher the titer was, the higher the transfection efficiency was. At 108 TU/mL lentiviral vectors, the cytotoxicity was increased although the transfection efficiency further elevated. Therefore, 107 TU/mL was defined as the optimal titer and the transfection efficiency was 85.87% at this titer. These findings suggested not only optimal suppression but safety should be taken into account in screening the optimal titers of lentiviral vectors aiming to decrease the potential side effects of lentiviral vectors. In the future animal experiments and clinical trials, the cytotoxicity of shRNA lentiviral vectors should be carefully assessed before they were used as drugs.Citation11
In our study, the interference efficiency of Col I shRNA lentiviral vectors in mesangial cells was investigated at the mRNA and protein levels. Results showed lentivirus-mediated Col I shRNA interference could efficiently inhibit mRNA expression of Col I. Furthermore, proteins were extracted from cells after 20 days of transfection and protein expression of Col I was also detected to explore whether Col I shRNA lentiviral vectors could persistently and stably exert suppressive effect, and whether lentiviral vectors were superior to other vectors (plasmid) in the interference efficiency.Citation12 Results showed the characteristics of Col I shRNA lentiviral vectors: continuity and stability.
Collagen type I is a protein secreted by mesangial cells after transformation and does not directly affect the growth of mesangial cells. However, in the RNAi, the interference genes carried by lentiviral vectors may influence the proliferation, apoptosis, and cell cycle to a certain extent.Citation13 In our study, the effects of blank lentiviral vectors and shRNA lentiviral vectors on mesangial cell proliferation were measured by MTT assay. Results showed lentivirus itself could inhibit mesangial cell proliferation and this suppressed proliferation was further enhanced by Col I shRNA lentiviral vectors. Thereafter, growth curves of mesangial cells were delineated, and results showed stable suppressive effects of blank lentiviral vectors and Col I shRNA lentiviral vectors (p > 0.05). In the occurrence and development of renal fibrosis, pathological hyperplasia of mesangial cells is a main presentation. Therefore, suppressed proliferation of mesangial cells by Col I shRNA lentiviral vectors may decrease the mesangial cells and reduce the production of Col I, leading to control of renal fibrosis.
Based on the results from apoptosis detection, lentivirus was cytotoxic and induced apoptosis of mesangial cells to a certain extent although it is a defective virus.Citation14 However, the cytotoxicity of lentiviral vectors carrying Col I shRNA was not markedly increased when compared with blank lentiviral vectors, suggesting favorable safety.
MTT assay displayed both blank lentiviral vectors and Col I shRNA lentiviral vectors conferred suppressive effects on the proliferation of rat mesangial cells. Which phase was inhibited and what was the potential mechanism were not clear. Thereafter, cell cycle was determined by flow cytometry. Results showed the proportion of cells in G2/M phase in pSC-GFP/Col I group and pSC-GFP group was higher than that in control group. This may be caused by lentivirus itself. That is to say, lentivirus arrested cells in G2/M phase. These findings implied lentivirus could inhibit cell dissociation in mitosis anaphase and subsequently suppress cell proliferation. After 72 h of transfection, numerous cells in pSC-GFP/Col I group were arrested in S phase which was consistent with the time course of gene transcription in RNAi. Moreover, proteins are mainly synthesized in S phase. Therefore, we could speculate the changes in cell cycle distribution was caused by Col I shRNA-induced suppression of Col I expression.
Col I shRNA arrested cells in S phase and subsequently decreased cell proliferation leading to the suppression of hyperplasia of mesangial cells. In addition, lentivirus arrested some cells in G2/M phase accompanied by slightly increased apoptosis due to its cytotoxicity, which was regarded as the side effect of lentivirus although the cytotoxicity was mild. The present study also investigated the way in which Col I shRNA lentiviral vectors were delivered. Reviewing existing literatures about RNAi may lead us to draw the conclusion that great achievements have been made on RNAi at cell level. However, in vivo application of RNAi technique is limited by several factors. How to deliver vehicles carrying shRNA is an unsolved problem in the field of RNAi and has been a hot topic in studies on RNAi.Citation15,Citation16 Recently, delivery of vehicles carrying siRNA to the kidney has been investigated and progress has been made.Citation17 In the present study, lentiviral vectors were injected into renal cortex. Results showed direct renal injection could efficiently deliver lentivirus to the kidney which has favorable targeting and reduces the side effects. These results may provide information for future animal studies.
Taken together, RNAi technique could stably and efficiently transfect Col I shRNA lentiviral vectors into mesangial cells, which subsequently inhibited Col I expression profoundly. Although certain side effects were observed, the overall safety was acceptable. Furthermore, renal injection of Col I shRNA lentiviral vectors was also feasible, which may provide theoretical basis for gene therapy of renal fibrosis.
ACKNOWLEDGMENTS
The authors thank Mr. Qianglin Duan from Tongji University for critical reading of the manuscript.
Declaration of interest: This work was supported by Beijing Natural Science Foundation (no. 7063094).
REFERENCES
- Zhou CH, Gao CF, Mei CL. Biological function and transcriptional regulation of collagen type I. Chin J Nephrol Dial Transplant. 2001;10(5):468–472.
- Ortega-Velazquez R, Gonzalez-Rubio M. Collagen I upregulates extracellular matrix gene expression and secretion of TGF-β1 by cultured human mesangial cells. Am J Physiol Cell Physiol. 2004;1:C1335–C1343.
- Lin SL, Kisseleva T, Brenner DA, Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am J Pathol. 2008;173(6):1617–1627.
- Shrey K, Suchit A, Nishant M, RNA interference: Emerging diagnostics and therapeutics tool. Biochem Biophys Res Commun. 2009;386(2):273–277.
- Krishnan P, Gireesh-Babu P, Rajendran KV, RNA interference-based therapeutics for shrimp viral diseases. Dis Aquat Organ. 2009;86(3):263–272.
- López-Fraga M, Martínez T, Jiménez A. RNA interference technologies and therapeutics: From basic research to products. BioDrugs. 2009;23(5):305–332.
- Vázquez-Vega S, Contreras-Paredes A, Lizano-Soberón M, RNA interference (RNAi) and its therapeutic potential in cancer. Rev Invest Clin. 2010;62(1):81–90.
- Tiemann K, Rossi JJ. RNAi-based therapeutics-current status, challenges and prospects. EMBO Mol Med. 2009;1(3):142–151.
- Shrey K, Suchit A, Nishant M, RNA interference: Emerging diagnostics and therapeutics tool. Biochem Biophys Res Commun. 2009;386:273–277.
- Sumimoto H, Kawakami Y. Lentiviral vector-mediated RNAi and its use for cancer research. Future Oncol. 2007;3:655–664.
- Lesch HP, Pikkarainen JT, Kaikkonen MU, Avidin fusion protein-expressing lentiviral vector for targeted drug delivery. Hum Gene Ther. 2009;20(8):871–882.
- Flotte TR. Gene therapy: The first two decades and the current state-of-the-art. J Cell Physiol. 2007;213(2):301–305.
- Yang M, Mattes J. Discovery, biology and therapeutic potential of RNA interference, microRNA and antagomirs. Pharmacol Ther. 2008;117:94–104.
- Lech P, Scmia NV. Retrovirus vectors. Contrib Nephrol. 2008;159(1):30–46.
- Singer O, Verma IM. Applications of lentiviral vectors for shRNA delivery and transgenesis. Curr Gene Ther. 2008;8(6):483–488.
- Morin A, Gallou-Kabani C, Mathieu JR, Systemic delivery and quantification of unformulated interfering RNAs in vivo. Curr Top Med Chem. 2009;9(12):1117–1129.
- Dissen GA, Lomniczi A, Neff TL, In vivo manipulation of gene expression in non-human primates using lentiviral vectors as delivery vehicles. Methods. 2009;49(1):70–77.