Abstract
Lipid deposition in glomerulus plays an important role in the progression of glomerulosclerosis (GS), and apolipoprotein E (apoE) is an important protein in cholesterol homeostasis. This investigation was performed to explore whether there exists an association between apoE and GS susceptibility. Eighty Wistar rats were randomly divided into two groups: sham operation group and glomerulosclerosis model group, n = 40. The GS disease in rat was induced by uninephrectomy and injecting adriamycin (5 mg/kg) through the tail vein. At the end of 9 and 13 weeks, 20 rats in each group were killed and the relative samples were collected. Serum creatinine, blood urea nitrogen, and 24-h urine protein were determined. Immunohistochemical analysis was performed on renal tissue to detect the expression of apoE, collagen IV, fibronectin, α-smooth muscle actin, and transforming growth factor-β1 in glomerulus. Real-time reverse transcription polymerase chain reaction was conducted to detect the apoE mRNA expression in renal tissue. Compared with sham operation group at the end of 9/13 weeks, glomerulosclerosis model group exhibited levels of serum creatinine, blood urea nitrogen, 24-h urine protein, and a glomerulosclerosis index that were significantly elevated ( p < 0.01), and collagen IV, fibronectin, α-smooth muscle actin, and transforming growth factor-β1 protein expression and apoE expression (protein and mRNA) were significantly upregulated (p < 0.01). In conclusion, apoE can increase the accumulation of extracellular matrix in glomerulus and may take part in the progression of GS.
INTRODUCTION
Apolipoprotein E (apoE), a 35-kDa plasma protein synthesized in liver and other organs such as kidney, regulates the lipid catabolism of triglyceride-depleted remnants of chylomicrons and very low density lipoprotein.Citation1,Citation2 Lipid deposition in glomerulus is an important characteristic of nephritic syndrome. The role of apoE in kidney has been controversial since the first investigation was reported. Investigators reported that the apoE took a protective role for kidney.Citation3,Citation4 Some other investigations found that the apoE expression in renal glomerulus was elevated when there were some renal diseases.Citation5–8 However, the available evidence reported to date is weak, due to small sample number in those studies to some extent, especially for some clinical studies. The investigation to explore the association of apoE with the accumulation of extracellular matrix (ECM) in glomerulosclerosis (GS) rats is rare. We performed this experiment to investigate the relation between apoE and GS in rats, with the intention to provide a much more reliable finding on the significance of the association.
MATERIALS AND METHODS
Animal Model
Eighty healthy male rats, 180–200 g, of Wistar backgrounds were purchased from the Experimental Animal Center of Guangxi Medical University, Nanning, China. All the procedures and protocols were approved by the Institutional Animal Care and Use Committee. The rats were divided into two groups randomly: sham operation group (SHO, n = 40) and GS model group without treatment (GS, n = 40). The SHO group was subjected to a sham operation and tail vein injection of normal saline solution alone. GS model in the GS group was induced by uninephrectomy, and adriamycin (ADR) (Wanle Pharmaceutical Co., Shenzhen, China) was injected in single tail vein after 1 week at a dose of 5 mg/kg, after dissolving in sterilized water. At the end of 9 and 13 weeks, 20 rats in each group were sacrificed; serum and urine were collected and stored at –20°C, and their kidneys were collected for histological and molecular biological determination.
At the end of 9 and 13 weeks, 24-h urine specimens were collected from 20 rats in each group. Blood specimens were collected before the rats were sacrificed. Serum creatinine (Scr) and blood urea nitrogen (BUN) levels were detected with a 7170-A biochemistry test kit (Hitachi Co., Tokyo, Japan). Urinary protein concentration from 24-h urine sample was measured by the sulfosalicylic acid method.
Renal Morphology
Renal tissues were fixed in 10% neutral formaldehyde, and they were dehydrated through a graded ethanol series and embedded in paraffin. Sections of 4 µm thickness were prepared on a microtome and stained with hematoxylin and eosin. Renal damage was viewed by light microscopy, and the severity of the renal lesion was defined by the glomerulosclerosis index (GSI). The GSI was calculated according to the method of Raij et al.Citation9 The severity of the lesions in a minimum of 40 glomeruli (range 40–80) in each specimen was examined, graded from 0 to 4 points in accordance with the percentage of morphological changes on each glomerulus (0 = 0%, 1 += 1–25%, 2 += 26–50%, 3 += 51–75%, 4 += 76–100%). The number of glomeruli showing a lesion of 0 was n0, of 1+ was n1, of 2+ was n2, of 3+ was n3, of 4+ was n4. Forty (range 40–80) glomeruli were examined independently, and the average score of each specimen was then calculated.
Immunohistochemical Analysis of apoE, Collagen IV, Fibronectin, α-Smooth Muscle Actin, and Transforming Growth Factor-β1
The operation was implemented according to the instructions of the SP staining system. Renal tissue samples were fixed in 10% neutral formaldehyde, dehydrated with ethanol, and embedded in paraffin. Serial 4 µm sections were collected sequentially on glass slides. The paraffin was removed from the sections with xylene and rehydrated in graded ethanol. To retrieve antigenicity from formalin fixation, we incubated the sections for 10 min in 10 mmol/L sodium citrate buffer using a microwave oven. Endogenous peroxidase activity was blocked by further pretreatment with 3% hydrogen peroxide (H2O2) and methanol. Finally, the sections were incubated with primary monoclonal antibodies against apoE (1/150) (Bo Ao-Sen, Co., Beijing, China), Collagen IV (Col-IV; 1/100) (Shanghai ChangDao Co., Shanghai, China), fibronectin (FN; 1/100) (Beijing Zhongshan Co. Ltd., Beijing, China), α-smooth muscle actin (α-SMA) (ready-to-use kit) (Shanghai Changdao, Co.), and transforming growth factor-β1 (TGF-β1; 1/100) (Wuhan Boshide, Co., Wuhan, China) overnight at 4°C. The sections were washed thoroughly in phosphate-buffered saline solution and incubated with rabbit anti-mouse biotinylated second antibody immunoglobulin (Shanghai Changdao, Co.) for 30 min. Finally, the sections were stained with diaminobenzidine. We obtained negative controls by replacing specific antisera with phosphate-buffered saline solution. For determination of the expression of apoE, Col-IV, FN, α-SMA, and TGF-β1, a semi-quantitative evaluation was performed. Score 0 represents little or no positive staining, whereas 1–4 represent the disruptive redistribution of apoE, Col-IV, FN, α-SMA, and TGF-β1, involving <25%, 25–50%, 50–75%, and >75% of positive staining of glomerular area, respectively.Citation10,Citation11 At least 40 randomly chosen glomeruli were evaluated at 400-folds original magnification for each mouse, and an average composite score was counted.
Real-Time Reverse Transcription Polymerase Chain Reaction to Detect apoE mRNA Expression
Renal tissue was homogenized and total RNA was extracted with TRIzol (Beijing Tiangen Co., Beijing, China). Ultraviolet spectrophotometer measured the absorbance, agarose gel electrophoresis confirmed that there had been no degradation of RNA. Primers for apoE and β-actin were designed according to primer design principles by Primer Premier 5.0. One microgram total RNA from the renal tissue of each rat was reverse transcribed into cDNA with an ExScript RT reagent kit (Takara Biotechnology Co., Dalian, China). ApoE was amplified with SYBR Premix Ex Taq (Beijing Tiangen Co.). The cycling parameters were denatured at 95°C for 5 s, with annealing at 61°C for 20 s and extension at 72°C for 15 s. A total of 35 cycles were performed within the linear amplification range. Gene expression of β-actin was also measured in each sample and used as an internal control for loading and reverse transcription efficiency. Each sample analysis was repeated three times. The average threshold cycle (CT, the cycles of template amplification to the threshold) was worked out as the value of each sample. Relatively quantitative 2−ΔΔCT was used to compare the mRNA expression.Citation12
Statistical Analysis
The data are shown as mean ± standard deviation (SD). The t-test for independent samples was performed to compare variables between groups, and Pearson's correlation coefficients were used to determine the relationships between the indicators for detection. A value of p < 0.05 was considered as significant. Statistical analysis was performed using the statistical package for social studies SPSS version 13.0 (SPSS, Chicago, IL, USA).
RESULTS
Changes in Renal Function
At the end of the 9/13 weeks, BUN and Scr levels of group GS were markedly higher than those in group SHO (p < 0.01). In GS group, BUN or Scr in 13 weeks was slightly increased compared with that of 9 weeks (BUN: 13/9 weeks = 1.25; Scr: 13/9 weeks = 1.15) ().
Table 1. Parameters in two groups (mean ± SD)
24-h Urinary Protein Excretion
When compared with SHO group, 24-h urine protein in GS showed a more significant elevation (p < 0.01), especially at the end of 13 weeks (GS group, 13/9 weeks = 1.30) ().
Glomerular Morphology
Light microscopy was performed to observe the changes of glomerular morphology. Glomerular morphologies were normal in sham group (A1 and A2; ). Extensive damage was observed in GS group, such as glomerular hypertrophy, balloon adhesion, and wall thickening (A3 and A4; ), especially at 13 weeks (A4; ). Quantitative analysis showed that the GSI of group GS at week 9/13 was much higher than that in SHO group (p < 0.01), and the GSI in 13 weeks in GS group was a little elevated when compared with that of 9 weeks (13/9 weeks = 1.34) ().
Figure 1. Glomerular morphology was normal in sham group (A1: 9 weeks; A2: 13 weeks; HE). Proliferation occurring in the majority of glomerular mesangial cells and extracellular matrix in GS group, and degeneration of glomerular epithelial cells and infiltration of widespread mononuclear cells were shown (A3: 9 weeks; A4: 13 weeks; HE), especially in 13 weeks of GS group (A4). Representative samples of immunohistochemical staining for glomerular Col-IV (SHO: B1: 9 weeks, B2: 13 weeks; GS: B3: 9 weeks and B4: 13 weeks), FN (SHO: C1: 9 weeks, C2: 13 weeks; GS: C3: 9 weeks and C4: 13 weeks), α-SMA (SHO: D1: 9 weeks, D2: 13 weeks; GS: D3: 9 weeks and D4: 13 weeks), TGF-β1 (SHO: E1: 9 weeks, E2: 13 weeks; GS: E3: 9 weeks and E4: 13 weeks), and apoE (SHO: F1: 9 weeks, F2: 13 weeks; GS: F3: 9 weeks and F4: 13 weeks) were observed in all groups. Sham group (B1, B2, C1, C2, D1, D2, E1, E2, F1, and F2): positive staining (in brown) was faint in glomerulus, glomerular endothelial cells, glomerular basement membrane, mesangial cells, and visceral epithelial cells. GS group (B3, B4, C3, C4, D3, D4, E3, E4, F3, and F4): positive staining was strong in most glomerulus, glomerular endothelial cells, glomerular basement membrane, mesangial cells, and visceral epithelial cells, especially in those of 13 weeks (B4, C4, D4, E4, and F4). Magnification 400×.
Notes: SHO, sham operation group; GS, glomerulosclerosis model group; HE, hematoxylin and eosin; FN, fibronectin; α-SMA, α-smooth muscle actin; TGF-β1, transforming growth factor-β1; apoE, apolipoprotein E.
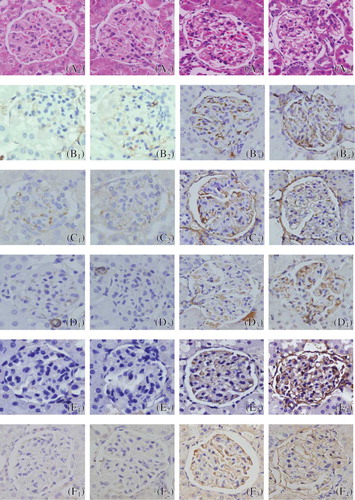
Col-IV, FN, α-SMA, and TGF-β1 Protein Expression in Glomerulus
Immunohistochemical staining for Col-IV, FN, α-SMA, and TGF-β1 was performed. Weak staining was observed in some glomerulus, glomerular endothelial cells, glomerular basement membrane, mesangial cells, and visceral epithelial cells of sham rats (B1, B2, C1, C2, D1, D2, E1, and E2; ). Staining was markedly enhanced in the majority of glomerulus, glomerular endothelial cells, glomerular basement membrane, mesangial cells, and visceral epithelial cells of GS rats (B3, B4, C3, C4, D3, D4, E3, and E4; ), especially for those in 13 weeks (B4, C4, D4, and E4; ). In the SHO group, the glomerular staining for Col-IV, FN, α-SMA, and TGF-β1 was markedly lower than the GS group (p < 0.01) in 9/13 weeks. Those of 13 weeks in GS group were remarkably elevated when compared with 9 weeks (Col-IV: 13/9 weeks = 1.40; FN: 13/9 weeks = 1.56; α-SMA: 13/9 weeks = 1.47; TGF-β1: 13/9 weeks = 1.42) ().
apoE Protein in Glomerulus and mRNA Expression of Renal Tissue
The apoE protein expressed in glomerular endothelial cells, glomerular basement membrane, mesangial cells, and visceral epithelial cells (). Control kidneys showed mild expression of apoE in glomerulus (F1 and F2; ). However, when compared to the kidneys of SHO, remarkable deposition of apoE was observed in the region of glomerular endothelial cells, mesangial cells, and visceral epithelial cells in GS (F3 and F4; ), especially in that of 13 weeks (F4; ). Consistently lower mRNA expression of renal tissue and alleviation of apoE staining in glomerulus in the SHO rats were observed when compared with those of GS rats at the end of 9/13 weeks (all p < 0.01), and those of 13 weeks in GS group were significantly increased when compared with 9 weeks (apoE protein: 13/9 weeks = 1.20; apoE mRNA: 13/9 weeks = 1.28) ().
Table 2. Protein expression of apoE in glomerulus and mRNA expression of apoE in renal tissue of the two groups (mean ± SD)
Correlation Analysis
Correlation analysis between apoE protein and Col-IV, FN, α-SMA, TGF-β1 protein or GSI in glomeruli was conducted for our investigation. Significant positive correlation was observed between apoE protein and Col-IV, FN, α-SMA, TGF-β1 protein, or GSI (r = 0.901, r = 0.893, r = 0.842, r = 0.826, r = 0.932, respectively; p < 0.01 for each).
DISCUSSION
Over-depositing of ECM such as FN and Col-IV is an important characteristic of GS. α-SMA is a specific marker for myofibroblasts and takes part in the development and progression of GS.Citation13,Citation14 Of all the cytokines and growth factors involved, TGF-β1 plays the most important role and the elevation of TGF-β1 expression is closely correlated with GS.Citation15,Citation16 TGF-β1 is known to be one of the major mediators that lead to GS by inducing the production of α-SMA and ECM (Col-IV and FN) in glomerulus.Citation15,Citation17,Citation18
In renal tissue, apoE is mainly synthesized by mesangial cell under normal physiologic conditions.Citation19 Investigators reported that the apoE took a protective role for kidney.Citation3,Citation4 Some other investigations found that the apoE expression in renal glomerulus was elevated when compared with that of the normal group;Citation5–7 however, those studies did not explore the relation between apoE and Col-IV, FN, TGF-β1, and α-SMA. The deposition of lipids in glomeruli, as an important pathogenetic factor, involves in glomerular sclerosis and leads to progression of renal disease. As a key apolipoprotein to mediate the catabolism of lipids deposition, the role and mechanism of apoE in GS is complicated, and there are rare reports that explore the association between apoE and the protein expression of Col-IV, FN, TGF-β1, or α-SMA. In this context, the aim of our study was to determine whether apoE expression correlates with the protein expression of Col-IV, FN, TGF-β1, and α-SMA in glomerulus and to compare these relationships with controls.
In this investigation, we found the expression of apoE in glomerulus might reflect the seriousness of GS in rats. The protein expressions of Col-IV, FN, α-SMA, and TGF-α1 in glomerulus were increased when compared with those in controls. The apoE protein expression in glomerulus of GS group was also much higher compared with that in SHO group. The correlation analysis was performed to explore the association between apoE protein and Col-IV, FN, α-SMA, TGF-β1 protein, or GSI in glomeruli and significant positive correlation was observed between apoE protein and Col-IV, FN, α-SMA, TGF-β1 protein, or GSI. The expression of apoE mRNA was elevated in GS when compared with that in control group. We also measured the protein expression of apoE in tubular interstitium and found the apoE expression in tubular interstitium was less when compared with that in glomerulus in SHO group or group of GS (data not shown). In other words, the increased apoE expression in glomerulus was more remarkable than that in tubular interstitium. So, in disease state of GS, the elevated expression of apoE mRNA in renal tissue might be mainly contributed by the glomerulus. We speculated that the apoE was a risk factor for GS, but the role and mechanism of apoE in the pathological process of GS was ambiguous.
Endothelial, mesangial, and visceral epithelial cells in glomerulus can take up the lipids by receptor-independent mechanism. Those lipoproteins could be rapidly removed by the glomerular cells and lead to lipid deposition in glomerulus.Citation20 apoE, as an important apolipoprotein, mediates the lipoproteins combining with the glomerular cells for catabolism. However, the over-upgraded apoE mediates too much lipid depositing in glomerular cells which also can cause the progression of GS. In our study, we found the apoE was much markedly accumulated in glomerular of GS rats when compared with that in SHO. We draw a conjecture that the apoE mediated the lipoproteins integrating with endothelial, mesangial, and visceral epithelial cells and induced the accumulation of lipids in glomerulus. The direct negative effects of lipid over-accumulation on the renal parenchymal cells was due to the formation of free radicals because of oxidized low-density lipoprotein (LDL),Citation7 and oxidized LDL can induce mesangial cells and macrophages to release cytokines and growth factors (such as TGF-β1), which may increase mesangial proliferationCitation21,Citation22 and lead to ECM accumulation and α-SMA over-expression. The role of apoE taking part in the pathological mechanism of GS might be that the apoE mediated too much lipoprotein depositing in glomerulus and leaded to GS.
How to regress or repair the GS is a difficult problem to solve at present. There were some reports observed that some new agents, such as angiotensin II blockers, all-trans retinoic acid, and so on, could alleviate the pathological changes of ADR-induced GS in the past years.Citation23–25 Statins, as an important agent to mediate the lipid metabolism, can reduce renal injury via diminishing the proliferation of mesangial and vascular smooth muscle cells, and inhibiting macrophage infiltration and the production of cytokines.Citation26 Some investigations have found that statins can play a protective role in patients with renal disease or animal model accompanying renal lesion.Citation25,Citation27,Citation28 Whether the statins can alleviate the lesion of GS by regulating the expression of apoE, it might be an interesting issue to be explored in times to come.
In conclusion, although the relationship between apoE and GS is till now not fully elucidated, these observations in our study may suggest that apoE is actively involved in the pathogenesis of GS by upregulating the protein expressions of Col-IV, FN, TGF-β1, and α-SMA in glomerulus in rats. Considering the widespread interest in apoE and their interactions in the field of GS, our investigation might have significant influence on future research as well as making some appropriate interventions for GS. However, more studies by cell culture or other experimental models of renal disease are needed to explore the molecular mechanisms and confirm the role of apoE in the pathogenesis of GS in the future.
ACKNOWLEDGMENTS
This study was supported by the Natural Science Foundation of the Guangxi Zhuang Autonomous Region (no. 0640103) and the Education Department of Guangxi Zhuang Autonomous Region (no. 0810). The authors gratefully acknowledge the most helpful comments on this article received from Professor Liang Rong, Department of Pediatric-Neonatology, Baylor College of Medicine, Houston, TX, USA.
Declaration of interest: The authors report no conflicts of interest. The author alone is responsible for the content and writing of the paper.
REFERENCES
- Attila G, Noyan A, Karabay BA, Acarturk E, Anarat A. Apolipoprotein E polymorphism in childhood nephrotic syndrome. Pediatr Nephrol. 2002;17:359–362.
- Hu P, Qin YH, Lu L, Hu B, Jing CX, Lei FY. Genetic variation of apolipoprotein E does not contribute to the lipid abnormalities secondary to childhood minimal change nephrotic syndrome. Int Urol Nephrol. 2010;42:453–460.
- Chen G, Paka L, Kako Y, Singhal P, Duan W, Pillarisetti S. A protective role for kidney apolipoprotein E. Regulation of mesangial cell proliferation and matrix expansion. J Biol Chem. 2001;276:49142–49147.
- Zhang Y, Yasumoto Y, Ikeda T, Takenouchi S, Sogabe A, Nosaki T. Apolipoprotein E regulates primary cultured human mesangial cell proliferation. Nephron Exp Nephrol. 2006;102:62–70.
- Calandra S, Gherardi E, Fainaru M, Guaitani A, Bartosek I. Secretion of lipoproteins, apolipoprotein A-I and apolipoprotein E by isolated and perfused liver of rat with experimental nephrotic syndrome. Biochim Biophys Acta. 1981;665:331–338.
- Deighan CJ, Caslake MJ, McConnell M, Boulton-Jones JM, Packard CJ. Patients with nephrotic-range proteinuria have apolipoprotein C and E deficient VLDL1. Kidney Int. 2000;58:1238–1246.
- Russi G, Furci L, Leonelli M, Magistroni R, Romano N, Rivasi P. Lipoprotein glomerulopathy treated with LDL-apheresis (heparin-induced extracorporeal lipoprotein precipitation system): A case report. J Med Case Rep. 2009;3:9311.
- Saito T, Sato H, Oikawa S. Lipoprotein glomerulopathy: A new aspect of lipid induced glomerular injury. Nephrology. 1995;1:17–24.
- Raij L, Azar S, Keane W. Mesangial immune injury, hypertension, and progressive glomerular damage in Dahl rats. Kidney Int. 1984;26:137–143.
- Dai C, Stolz DB, Bastacky SI, St-Arnaud R, Wu C, Dedhar S. Essential role of integrin-linked kinase in podocyte biology: Bridging the integrin and slit diaphragm signaling. J Am Soc Nephrol. 2006;17:2164–2175.
- Hu P, Qin YH, Pei J, Lei FY, Hu B, Lu L. Beneficial effect of all-trans retinoic acid (ATRA) on glomerulosclerosis rats via the down-regulation of the expression of alpha-smooth muscle actin: A comparative study between ATRA and benazepril. Exp Mol Pathol. 2010;89:51–57.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 2001;25:402–408.
- Danilewicz M, Wagrowska-Danielwicz M. Morphometric and immunohistochemical insight into focal segmental glomerulosclerosis in obese and non-obese patients. Nefrologia. 2009;29:35–41.
- Kaouthar M, Faical J, Abdelmajid K, Basma H, Mohamed BH, Hafedh M. Renal α-smooth muscle actin: A new prognostic factor for lupus nephritis. Nephrology. 2009;14:499–505.
- Wang W, Liu F, Chen N. Peroxisome proliferator-activated receptor-gamma (PPAR-gamma) agonists attenuate the profibrotic response induced by TGF-beta1 in renal interstitial fibroblasts. Mediat Inflamm. 2007;2007:62641.
- Adan VS, Javier AM, Marisol R, Jaime GM, Dolores UB, Rita GD. Association of polymorphisms within the transforming growth factor-b1 gene with diabetic nephropathy and serum cholesterol and triglyceride concentrations. Nephrology. 2010; 15:644–648.
- Yutaka K, Atsuhito N, Chisei RA, Ko O, Eri M, Hideoki O. Role of transforming growth factor-β/Smad signalling pathway in enhanced production of glomerular extracellular matrix in IgA nephropathy of high-serum-IgA ddY mice. Nephrology. 2002; 7:151–155.
- Hakki A, Mehmet K, Fulya YC, Serhan T, Cetin O, Akoglu E. Histopathological changes and tumour necrosis factor-α, transforming growth factor-β and tenascin expression in patients with primary type I membranoproliferative glomerulonephritis in remission. Nephrology. 2009;14:219–226.
- Lin CT, Xu YF, Wu JY, Chan L. Immunoreactive apolipoprotein E is a widely distributed cellular protein. Immunohistochemical localization of apolipoprotein E in baboon tissues. J Clin Invest. 1986;78:947–958.
- Grone HJ, Walli AK, Grone E, Kramer A, Clemens MR, Seidel D. Receptor mediated uptake of apo B and apo E rich lipoproteins by human glomerular epithelial cells. Kidney Int. 1990;37:1449–1459.
- Kaysen GA, der de Sain-van VM. New insights into lipid metabolism in the nephrotic syndrome. Kidney Int Suppl. 1999;71:18–21.
- Kamanna VS, Bassa BV, Ganji SH. Low density lipoproteins transactivate EGF receptor: Role in mesangial cell proliferation. Life Sci. 2008;83:595–601.
- Hayashi K, Sasamura H, Ishiguro K, Sakamaki Y, Azegami T, Itoh H. Regression of glomerulosclerosis in response to transient treatment with angiotensin II blockers is attenuated by blockade of matrix metalloproteinase-2. Kidney Int. 2010;78:69–78.
- Qin YH, Lei FY, Hu P, Pei J, Feng ZB, Pang YS. Effect of all-trans retinoic acid on renal expressions of matrix metalloproteinase-2, matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 in rats with glomerulosclerosis. Pediatr Nephrol. 2009;24:1477–1486.
- Zhang W, Li Q, Wang L, Yang X. Simvastatin ameliorates glomerulosclerosis in adriamycin-induced-nephropathy rats. Pediatr Nephrol. 2008;23:2185–2194.
- Zhou MS, Schuman IH, Jaimes EA, Raij L. Renoprotection by statins is linked to a decrease in renal oxidative stress, TGF-beta, and fibronectin with concomitant increase in nitric oxide bioavailability. Am J Physiol Renal Physiol. 2008;295:53–59.
- Chade AR, Zhu XY, Grande JP, Krier JD, Lerman A, Lerman LO. Simvastatin abates development of renal fibrosis in experimental renovascular disease. J Hypertens. 2008; 26:1651–1660.
- Garcia-de-la-Puente S, Arredondo-Garcia JL, Gutierrez-Castrellon P, Bojorquez-Ochoa A, Maya ER, Perez-Martinez MP. Efficacy of simvastatin in children with hyperlipidemia secondary to kidney disorders. Pediatr Nephrol. 2009;24:1205–1210.
