Abstract
Background: The effect of corticosteroids on renal cholesterol crystal embolism (CCE) remains uncertain. The aim of the present study was to elucidate the effect of steroid therapy on short- and long-term renal outcome in CCE patients. Methods: Fifty-one patients diagnosed with renal CCE were included in this retrospective study. The patients were divided into two groups according to whether or not they had received steroid therapy (steroid therapy (+), n = 32; (–), n = 19). Corticosteroids were administered at an initial dose of 10–20 mg/day after CCE diagnosis. The values of the estimated glomerular filtration rate (eGFR) in the two groups were examined at CCE diagnosis, 4 weeks after diagnosis and the last follow-up. Additionally, the % change in eGFR at 4 weeks after diagnosis and % change per year in eGFR at the last follow-up were calculated for each patient. Results: The median values of eGFR at diagnosis in patients with and without steroid therapy were 16.4 and 17.9 mL/min/1.73 m2, respectively. The median % change in eGFR between diagnosis and 4 weeks after diagnosis was 24% in patients with steroid therapy and 5% in those without, and this difference was statistically significant. On the other hand, there was no significant difference between the two groups in the % change in eGFR per year between diagnosis and the last follow-up. Conclusions: During the short period after CCE diagnosis, steroid therapy showed a good renal outcome in CCE patients. However, this treatment did not have a favorable effect on long-term renal outcome.
INTRODUCTION
Cholesterol crystal embolism (CCE) is a systemic disease resulting from cholesterol crystal embolization and leading to the occlusion of small vessels in a variety of organs, including the kidneys, skin, brain, gastrointestinal tract, and extremities.Citation1 When the clinical manifestations of CCE are limited to the lower extremities, the clinical course seems to be relatively benign. However, CCE can also result in multiple organ failure, including renal failure, cardiac failure, gastrointestinal involvement, pancreatitis, encephalopathy, muscle ischemia, and skin necrosis, and in such cases it is a life-threatening condition.Citation2–8
Clinically, renal involvement (renal CCE) occurs in approximately 50% of CCE patients.Citation2,Citation9 Since the 1990s, renal CCE has been recognized as a cause of renal deterioration in elderly patients with remarked atherosclerosis.Citation3,Citation5–8 The prognosis of renal CCE is considered poor. Various treatments for renal CCE, such as corticosteroids, low-density lipoprotein apheresis, and statins, have been attempted. However, no optimal or standardized therapy for CCE has been established. High-dose corticosteroids, including pulse therapy, have resulted in dramatic improvement of kidney function in some cases.Citation10–12 Previous reports demonstrated that corticosteroids at a dose of 15 or 20 mg/day ameliorate acute aggravation of kidney function caused by CCE.Citation13–15 We also reported the efficacy of low-dose corticosteroids on acute renal deterioration due to CCE.Citation16 However, these previous reports were conducted in small samples. In contrast, there have been case reports in which steroid therapy for CCE had limited or no effect.Citation2,Citation17,Citation18 Furthermore, in renal CCE patients, very few studies have addressed the efficacy of steroid therapy on both short- and long-term renal outcome, and there has been no published report comparing renal outcome between subjects with and without steroid therapy. Therefore, the role of corticosteroids in treating renal CCE has remained controversial.
The present retrospective study was based on our data of 51 Japanese patients with CCE, who presented with acute/subacute or chronic renal deterioration and were treated at a single center. The clinical features and renal outcomes were examined. Patients were subdivided according to whether or not they were administered low-dose corticosteroids for renal CCE. By retrospectively comparing the short- and long-term renal outcome in CCE patients with and without steroid therapy, we aimed to elucidate whether steroid therapy has an effect on renal outcome either immediately after CCE diagnosis or over the long term.
PATIENTS AND METHODS
Between November 2000 and February 2009, we encountered 61 Japanese patients who presented with livedo reticularis and/or blue toes, eosinophilia, and acute/subacute or chronic renal deterioration, and who were admitted to our hospital. These patients were diagnosed as having CCE based on clinical and/or pathological findings. Of the 61 patients, 10 developed end-stage renal disease and/or died within 4 weeks after CCE diagnosis, and the remaining 51 were enrolled in the present retrospective study. In all patients, contrast media-induced nephropathy, perioperative or postoperative hemodynamic instability, or drug-induced nephrotoxicity, including that from angiotensin-converting enzyme inhibitors and/or angiotensin II receptor blockers and nonsteroidal anti-inflammatory drugs, were not considered a cause of renal deterioration based on clinical grounds. Data were retrospectively collected for each patient. All of the patients provided written informed consent, which was approved by the Ethics Committee of this institution. Preliminary data on 7 of the 51 patients have been reported previously.Citation16 Clinical data as well as the presence of precipitating factors were recorded at the time of CCE diagnosis. The estimated glomerular filtration rate (eGFR) (mL/min/1.73 m2) was calculated by the Modification of the Diet in Renal Disease equation for Japanese patients: 194 × serum creatinine (SCr)−1.094 × age−0.287 × 0.739 (if female).Citation19 The lowest SCr value within the 8-week period before CCE diagnosis was recorded for each patient, and then the value of eGFR calculated using this SCr level was defined as the baseline eGFR. In a similar fashion, the lowest eosinophil count and C-reactive protein in the 8 weeks before diagnosis were defined as the baseline eosinophil count and C-reactive protein, respectively. SCr, eGFRs, eosinophil counts, and C-reactive protein 4 weeks after CCE diagnosis or at the last follow-up in each patient were recorded. Eosinophilia was defined as an absolute count >500 per µL.
Acute/subacute renal deterioration was diagnosed when the value of eGFR at diagnosis decreased by more than 50% compared with the baseline eGFR, whereas chronic renal deterioration was diagnosed when the value of eGFR at diagnosis decreased by less than 50% compared with the baseline eGFR. The diagnosis of iatrogenic CCE was made in atherosclerotic patients with renal deterioration in the presence of one or more of the following precipitating factors: arterial angiography with or without angioplasty, vascular surgery such as coronary artery bypass graft, abdominal aortic graft surgery and carotid endarterectomy, and anticoagulant therapy (use of heparin or oral vitamin K antagonists). In contrast, renal CCE was defined as a spontaneous form without the above precipitating factors.
Demographic information (age and gender), comorbidities (ischemic heart disease, cerebrovascular disease, aortic aneurysm, and peripheral artery disease), and atherosclerotic risk factors (hypertension, history of smoking, dyslipidemia, and diabetes mellitus) at CCE diagnosis were recorded for each patient. Angiotensin-converting enzyme inhibitors and/or angiotensin II receptor blockers and statins which had been previously prescribed for each patient were reviewed before the diagnosis of CCE.
Informed consent for steroid therapy was obtained from each patient. The decision as to whether or not to use corticosteroids was made on an individual basis without any specific criteria, based on the clinical background and the patient's acceptance. Steroid therapy at a dose of 10–20 mg/day was started after CCE diagnosis. Thereafter, corticosteroids were tapered at a rate of 5 mg/day at an interval of 2–4 weeks. Finally, corticosteroids were administered at a maintenance dose of 2.5–5 mg/day. The median duration of the protocol was 11.6 months. To elucidate the effects of steroid therapy on short- and long-term renal outcome, we calculated the % change in SCr, eGFR, eosinophil counts, or C-reactive protein at 4 weeks after diagnosis or per year at the last follow-up from diagnosis, and then compared it with the % changes in patients with and without steroid therapy. The % changes in SCr, eGFR, eosinophil counts, or C-reactive protein 4 weeks after diagnosis were calculated using the following formula: [SCr, eGFR, eosinophil counts, or C-reactive protein (4 weeks) – SCr, eGFR, eosinophil counts, or C-reactive protein (diagnosis)/SCr, eGFR, eosinophil counts, or C-reactive protein (diagnosis)] × 100 (%). The % changes in SCr, eGFR, eosinophil counts, or C-reactive protein per year at the last follow-up were calculated using the following formula: [SCr, eGFR, eosinophil counts, or C-reactive protein (last follow-up) – SCr, eGFR, eosinophil counts, or C-reactive protein (diagnosis)/SCr, eGFR, eosinophil counts, or C-reactive protein (diagnosis)]/follow-up period (years) from diagnosis × 100 (%).
STATISTICAL ANALYSIS
Continuous data are expressed as either the mean ± standard deviation (SD) or median [interquartile range (IQR)] depending on the data distribution, and categorical data are expressed as numbers (with %). Differences in the prevalence were evaluated using the chi-square test and Fisher's exact test of groups containing less than five individuals in any given cell. The statistical significance of differences between groups was examined using the Wilcoxon rank sum test for nonparametric data or unpaired Student's t-test for parametric data. Pearson's correlation coefficient was used to evaluate whether the % changes in eGFR from baseline to diagnosis were correlated with those in eosinophil counts or C-reactive protein; the % changes in eGFR, eosinophil counts, or C-reactive protein were calculated by the formula [eGFR, eosinophil counts, or C-reactive protein (diagnosis) – eGFR, eosinophil counts, or C-reactive protein (baseline)/eGFR, eosinophil counts, or C-reactive protein (baseline)] × 100 (%), and the analysis was performed using log-transformed values to approximate a normal distribution. The Wilcoxon signed rank test was used to analyze the change of SCr or eGFR from diagnosis to 4 weeks in each patient in the two groups with and without steroid therapy. Data were statistically analyzed using JMP8 (SAS Institute, Cary, NC, USA). A p-value below 0.05 indicated a statistically significant difference.
RESULTS
shows the clinical characteristics in the 51 CCE patients (males 45; females 6). The mean age was 73.8 ± 6.8 years with a range from 57 to 88 years. Forty-seven patients (92%) showed livedo reticularis and/or blue toes. Of these 47 patients, 34 with cutaneous involvement received a skin biopsy, and 16 of these 34 patients showed cholesterol clefts in the dermis. In the remaining 18 patients, although cholesterol clefts were not detected in the dermis, dilatation of the small arteries with reactive intimal swelling was observed, which is suspicious of CCE. One patient without cutaneous involvement received a renal biopsy, which showed needle-shaped cholesterol crystals in an interlobular artery. Iatrogenic CCE with precipitating factors such as angiography and/or cardiovascular surgery, or anticoagulant therapy was seen in 32 patients (63%). In 27 patients, anticoagulant therapy had been commenced before CCE diagnosis. After CCE diagnosis, 22 of these patients received no anticoagulant agents, whereas in the remaining 5 anticoagulant therapy with oral vitamin K antagonists was continued because of the presence of atrial or ventricular thrombus, intra-aortic mobile plaque, atrial fibrillation, or a postoperative state of below-knee graft bypass. Spontaneous CCE was found in 19 patients (37%). Acute/subacute renal deterioration due to CCE was observed in 13 patients. The 51 patients were divided into two groups: 32 patients with steroid therapy and 19 without. With regard to clinical characteristics, there was no significant difference in age or the prevalence of male gender, hypertension, or diabetes mellitus between the two groups. The prevalence of acute/subacute renal deterioration or iatrogenic CCE also did not differ between the two groups. The prevalence of ischemic heart disease in patients with steroid therapy was significantly lower compared with that in patients without, and the number of patients who had previously been administered statins before CCE diagnosis was higher in the group with steroid therapy than in that without.
Table 1. Clinical characteristics in the 51 patients with cholesterol crystal embolism
Data for eGFR and eosinophil counts were not obtained at 4 weeks after diagnosis for one patient without steroid therapy, and those at the last follow-up were not obtained for one patient with steroid therapy. In two patients without steroid therapy, C-reactive protein was not obtained at 4 weeks, and it was not monitored in one patient without steroid therapy at the last follow-up.
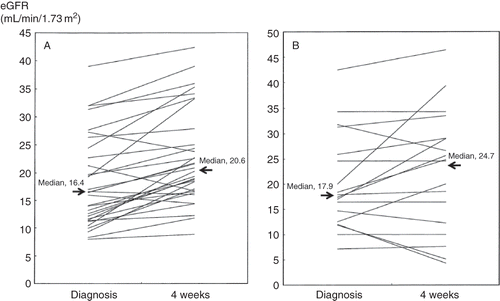
shows the renal or patient outcome in the 51 CCE patients. In the total patient group, the median values of SCr or eGFR at baseline were 2.0 mg/dL or 26.6 mL/min/1.73 m2, respectively, and at diagnosis, 2.9 mg/dL or 17.0 mL/min/1.73 m2, respectively. The use of statins, which included both continued and newly started administration after CCE diagnosis, was observed in 71% of all patients. At both baseline and diagnosis, there was no difference in SCr or eGFR levels between the two groups. Although the SCr or eGFR level at 4 weeks after diagnosis in patients with steroid therapy was not significantly different from that in patients without steroid therapy, the median % changes in SCr or eGFR in the former group was significantly decreased or increased, respectively, compared with those in the latter group. Furthermore, as shown in , using the Wilcoxon matched pairs signed rank test, in patients with steroid therapy, the values of eGFR 4 weeks after diagnosis were significantly increased compared with those at diagnosis (p < 0.0001), whereas no significant difference was found in the eGFR levels at diagnosis and 4 weeks after diagnosis in patients without steroid therapy (p = 0.19). In a similar fashion, SCr 4 weeks after diagnosis was significantly decreased compared with that at diagnosis in patients treated with corticosteroids (p < 0.0001), whereas no significant change of SCr from diagnosis to 4 weeks was found in patients without corticosteroids treatment (p = 0.43). On the other hand, there was no significant difference in the value of SCr or eGFR or the % changes per year in either SCr or eGFR at the last follow-up between the two groups, as shown in . With regard to body mass index at all stages, there was no significant difference between the two groups with and without steroid therapy. Despite the absence of the administration of both corticosteroids and statins, four patients showed an increase in eGFR levels both at 4 weeks and at last follow-up compared with those at CCE diagnosis. One patient without steroid therapy recovered kidney function after 6 weeks of hemodialysis.
Table 2. Renal or patient outcome in the 51 patients with cholesterol crystal embolism
Figure 1. The change of eGFR from diagnosis to 4 weeks after CCE diagnosis in patients with (A) and without steroid therapy (B). Note: eGFR, estimated glomerular filtration rate; CCE, cholesterol crystal embolism.
shows the time course of eosinophil counts and C-reactive protein in the 51 CCE patients. The median eosinophil count at diagnosis was 677 per µL, and the prevalence of eosinophilia was 78% at that time point. In addition, the % changes in eGFR from baseline to diagnosis negatively correlated with those in eosinophil counts and those in C-reactive protein during the same period (r = –0.45, n = 48, p = 0.002; r = –0.30, n = 49, p = 0.03, respectively). At 4 weeks after diagnosis, a significant decrease in eosinophil counts in patients with steroid therapy was observed compared with patients without steroid therapy, and the median % changes in eosinophil counts in the former group (–83%; IQR, –90 to –65%) was significantly different from those in the latter group (–37%; IQR, –63 to –5%). However, no significant difference was found in the % change per year in eosinophil counts between the two groups. On the other hand, there was no significant difference in the % change at 4 weeks or % change per year at the last follow-up in C-reactive protein between the two groups.
Table 3. Time course of eosinophils and C-reactive protein in the 51 patients with cholesterol crystal embolism
We also compared the renal outcome in the two subgroups treated with statins alone or a combination of corticosteroids and statins. As shown in , in the 25 patients treated with the combination therapy, the median % change in eGFR at 4 weeks significantly increased, compared with that in 11 patients without. On the other hand, there was no significant difference in the % change per year in eGFR at the last follow-up between the two groups. One patient treated with a combination of corticosteroids and statins showed a re-increase in SCr level and eosinophil counts concomitant with exacerbation of cutaneous lesion under maintenance steroid therapy at a dose of 5 mg/day. Thereafter, a higher dose (20 mg/day) of corticosteroids was resumed, which resulted in an amelioration of kidney function 4 weeks after the start of the re-increasing dose.
Table 4. Comparison of renal outcome in 25 patients with combination of steroids and statins and 11 patients with statins alone
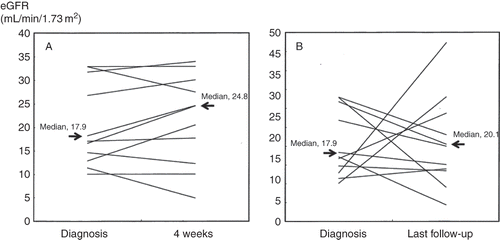
To elucidate the effect of statins on renal outcome throughout the entire course, we used the Wilcoxon matched pairs signed rank test to examine whether or not the change of eGFR from diagnosis to 4 weeks or last follow-up was statistically significant in 11 patients treated with statins alone. The results showed that eGFR at either 4 weeks or last follow-up did not change significantly compared with that at diagnosis (p = 0.36, p = 0.76, respectively, ).
Figure 2. The change of eGFR from diagnosis to 4 weeks (A) and last follow-up (B) in 11 CCE patients treated with statins alone. Note: eGFR, estimated glomerular filtration rate; CCE, cholesterol crystal embolism.
Low-dose corticosteroids were administered in 12 patients complicated with diabetes, 9 of whom showed poor glycemic control. One patient newly developed steroid-induced diabetes requiring insulin therapy. Two patients treated with corticosteroids died of pneumonia: one patient was given corticosteroids at an initial dose of 10 mg/day with a duration of 8.1 months and another at an initial dose of 15 mg/day with a duration of 0.5 months. In these cases, it was unclear whether corticosteroids contributed to the development of infection. In addition, one patient with steroid therapy died of lung cancer. This case had already been suggested of having malignancy before the initiation of steroid therapy, and this treatment thus did not affect the development of malignancy. The remaining patients experienced no major side effects, such as serious infection, gastrointestinal bleeding, or bone involvement, throughout the entire course.
DISCUSSION
Renal CCE is a frequently overlooked cause of kidney failure and has been dubbed a “silent masquerader.” It should be considered in the differential diagnosis in patients who present with progressive renal deterioration, particularly if they are elderly and have severe atherosclerosis.Citation20 CCE is increasingly recognized as an iatrogenic form arising after invasive manipulation of the aorta or large arteries, anticoagulant therapy, or fibrinolytic therapy, but it can also develop spontaneously.Citation2,Citation3,Citation5–8,Citation21 The presence of a triad composed of a precipitating event, acute or subacute renal failure, and peripheral CCE strongly suggests the diagnosis. In our series, the iatrogenic form was found in 32 patients (63%), which was lower than the prevalence (77–96%) reported in previous studies.Citation6–8 The most common manifestation in our study was cutaneous involvement (92%), as has been reported in the previous studies.Citation6,Citation8 The confirmation of CCE diagnosis is made by biopsy of the target organs, including kidney, skin, and muscle. During the acute phase of the disease, many patients may be too sick to receive a renal biopsy. In contrast, a skin biopsy is a simple and minimally invasive procedure. Therefore, generally, a skin biopsy should be considered first if the patients have visible skin lesions. In our series, one patient without cutaneous involvement received a renal biopsy, and 34 patients received a skin biopsy, half of whom were pathologically diagnosed with CCE, consistent with reports that the sensitivity of a skin biopsy specimen was 41–52%.Citation22,Citation23
Among the laboratory features, the presence of eosinophilia and increase in C-reactive protein are considered a helpful clue to the diagnosis of CCE.Citation5,Citation8,Citation24 A high prevalence of eosinophilia at CCE diagnosis was observed in our series as well. However, to date, very few reports have systemically described the time course of eosinophil counts and C-reactive protein. The present study is characterized by the description of eosinophil counts and C-reactive protein from a prediagnostic stage to last follow-up. It is of interest that negative correlations were found between the % changes in eGFR and those in eosinophil counts or C-reactive protein from baseline to diagnosis, suggesting that an increase in eosinophil counts or C-reactive protein might reflect the disease activity of renal CCE. In this context, a careful monitoring of eosinophil counts or C-reactive protein, including the prediagnostic level, could be useful in making the diagnosis of CCE or evaluating the degree of renal damage due to CCE.
Cholesterol emboli appear as needle-shaped clefts within the lumens of affected vessels. The subsequent intravascular inflammatory reaction causes tissue ischemia and ultimately leads to severe failure of various organs. The pathogenesis of renal failure due to CCE is thought to involve the reactive inflammation surrounding the cholesterol crystals, which leads to luminal occlusion. Peripheral eosinophilia also suggests a possible role for inflammation in the pathogenesis of renal CCE, which supports the rationale to use corticosteroids in this disease.Citation25 The involvement of immunologic mechanisms is also supported by clinical observations, such as the frequent appearance of eosinophilia.Citation2,Citation6–8 In the present study, patients treated with low-dose corticosteroids showed a significant improvement in renal outcome during the short period after CCE diagnosis compared with those not so treated. Simultaneously, eosinophil counts dramatically decreased in patients with steroid therapy compared with patients without steroid therapy. Based on these findings, the effect of corticosteroids seems attributable to the reduction of the inflammatory reaction after cholesterol crystals of plaques lodge to the peripheral arteries. It is also suggested that even if an initial dose of corticosteroids is low, the treatment could be sufficient to reduce the inflammatory reaction during the short period after CCE diagnosis. On the other hand, the % change in C-reactive protein at 4 weeks did not differ between the groups with and without steroid therapy, suggesting that eosinophil counts could be more sensitive to steroid therapy compared with C-reactive protein, during the short period after CCE diagnosis. Additionally, the % changes per year at the last follow-up in SCr or eGFR were shown to be almost equal between the groups with and without steroid therapy, although those in eosinophil counts or C-reactive protein in patients without steroid therapy tended to be higher compared with those in patients with steroid therapy. One possible explanation for this discrepancy is that the maintenance dose of corticosteroids in this study may have contributed to the decrease in eosinophil counts or C-reactive protein at the last follow-up, but may not have had a favorable effect on the long-term renal outcome. This observation may indicate that the decrease in eosinophil counts or C-reactive protein by steroid therapy is not necessarily associated with the improvement of renal function. Obviously, further studies are necessary to investigate the relationship between the changes of eosinophil counts or C-reactive protein and renal outcome.
Scolari et al. have reported that statins have the potential ability to stabilize cholesterol-rich aortic atherosclerotic plaques, which can shower cholesterol emboli into both the renal and pedal circulations, having a favorable effect on the renal or patient outcome.Citation6,Citation7 In the present study, treatment with statins alone in 11 patients failed to show any protective effect on renal outcome. However, considering the small size of our sample, we cannot rule out the possibility that statins have the ability to ameliorate renal damage due to CCE.
Several reports have demonstrated an improvement of kidney function by administration of steroids and worsening of kidney function after steroid withdrawal.Citation10,Citation11,Citation26 At the last follow-up, there was no significant difference in the % changes per year in SCr or eGFR between the groups with and without steroid therapy. In many of the previously reported cases, tapering of corticosteroids resulted in progression of the disease and increasing the dose or resuming the drug was required.Citation10,Citation11,Citation26 This pathogenesis might involve two mechanisms: CCE has been shown to be strictly correlated with the severity of atherosclerotic aortic plaque erosionCitation27; and the recurrent or continuous delivery of cholesterol crystals from severe or large ulcerated plaques might contribute to the re-exacerbation of renal function after corticosteroids are tapered or stopped. The lack of effectiveness of steroid therapy on long-term renal outcome in the current study might be attributed to the insufficient maintenance dose or duration of the therapy. Accordingly, a large prospective study will be warranted to determine the ideal maintenance dose or duration of steroid therapy to best improve long-term renal outcome in CCE patients.
In CCE patients, spontaneous recovery of kidney function is one of the clinical features. In our series, some patients showed recovery of kidney function after CCE diagnosis without corticosteroid or statin treatment, and in one patient kidney function recovered 6 weeks after the initiation of hemodialysis. The previous reports also suggest the possibility of spontaneous recovery of kidney function in approximately one-third of patients, even after variable periods of dialytic support.Citation3,Citation6–9 Several factors, such as reversal of inflammation, resolution of acute tubular necrosis in ischemic areas, and hypertrophy in surviving nephrons, seem to be the mechanisms causing the spontaneous recovery of kidney function.Citation15 In addition, it is important to note that spontaneous recovery, as well as a favorable renal outcome induced by steroid therapy, is possible when the aortic plaque is small or absent and the shower of cholesterol crystals stops. It would be helpful to decide the indication of corticosteroids if the predictive factors for spontaneous recovery could be clarified.
The present study had some important limitations which bear mention. This was a retrospective study, dependent on the decision of individual physicians as to whether corticosteroids should be administered, and the protocol of steroid therapy for each patient varied with regard to the dose or duration of corticosteroids. Therefore, our results on the effect of steroid therapy on renal outcome could be biased. As many patients in our series were given statins, the effect of statins on renal outcome could not be fully excluded. Nevertheless, the result that the combination therapy with corticosteroids and statins showed a more favorable effect on short-term renal outcome compared with statins alone in the subgroup analysis might highlight the efficacy of steroid therapy. In this context, large prospective studies, including a randomized controlled study – for example, a study comparing the efficacy of statins versus the combination of statins plus corticosteroids – will be warranted to clarify the efficacy of steroid therapy on renal outcome in CCE patients.
In conclusion, immediately after CCE diagnosis, low-dose corticosteroids had a favorable renal outcome in CCE patients who had not developed end-stage renal disease or death shortly after the diagnosis of CCE. However, this therapy was not associated with a favorable long-term renal outcome, suggesting that the maintenance dose or duration of corticosteroids may have been inadequate.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Scoble JE, O'Donnell PJ. Renal atheroembolic disease: The Cinderella of nephropathy? Nephrol Dial Transplant. 1996;11:1516–1517.
- Fine MJ, Kapoor W, Falanga V. Cholesterol crystal embolization: A review of 221 cases in the English literature. Angiology. 1987;38:769–784.
- Thadhani RI, Camargo CA, Jr, Xavier RJ, Fang LS, Bazari H. Atheroembolic renal failure after invasive procedures: Natural history based on 52 histologically proven cases. Medicine. 1995;74:350–358.
- Dahlberg PJ, Frecentese DF, Cogbill TH. Cholesterol embolism: Experience with 22 histologically proven cases. Surgery. 1989;105:737–746.
- Scolari F, Tardanico R, Zani R, Cholesterol crystal embolism: A recognizable cause of renal disease. Am J Kidney Dis. 2000;36:1089–1109.
- Scolari F, Ravani P, Pola A, Predictors of renal and patient outcomes in atheroembolic renal disease: A prospective study. J Am Soc Nephrol. 2003;14:1584–1590.
- Scolari F, Ravani P, Gaggi R, The challenge of diagnosing atheroembolic renal disease. Clinical features and prognostic factors. Circulation. 2007;116:298–304.
- Belenfant X, Meyrier A, Jacquot C. Supportive treatment improves survival in multivisceral cholesterol crystal embolism. Am J Kidney Dis. 1999;33:840–850.
- Scolari F, Bracchi M, Valzorio B, An increasingly recognized cause of acute renal failure. Nephrol Dial Transplant. 1996;11:1607–1612.
- Fabbian F, Catalano C, Lambertini D, Bordin V, Di Landro D. A possible role of corticosteroids in cholesterol embolization. Nephron. 1999;83:189–190.
- Graziani G, Santostasi S, Angelini C, Badalamenti S. Corticosteroids in cholesterol emboli syndrome. Nephron. 2001;87:371–373.
- Mann SJ, Sos TA. Treatment of atheroembolization with corticosteroids. Am J Hypertens. 2001;14:831–834.
- Daimon S, Motita R, Ohtsuki N, LDL apheresis followed by corticosteroid therapy as a possible treatment of cholesterol crystal embolism. Clin Exp Nephrol. 2000;4: 352–355.
- Nakahama H, Sakaguchi K. Small dose oral corticosteroid treatment rapidly improved renal function in a patient with acute aggravation of chronic renal failure due to cholesterol embolism. Nephrol Dial Transplant. 2001;16:872–873.
- Tamura K, Umemura M, Yano H, Acute renal failure due to cholesterol crystal embolism treated with LDL apheresis followed by corticosteroid and candesartan. Clin Exp Nephrol. 2003;7:67–71.
- Nakayama M, Nagata M, Hirano T, Low-dose prednisolone ameliorates acute renal failure caused by cholesterol crystal embolism. Clin Nephrol. 2006;66:232–239.
- Kaufman JL, Stark K, Brolin BE. Disseminated atheroemboli from extensive degenerative atherosclerosis of aorta. Surgery. 1987;102:63–70.
- Colt HG, Begg RJ, Saporito JJ, Cooper WM, Shapiro AP. Cholesterol emboli after cardiac catheterization. Eight cases and a review of the literature. Medicine. 1988;67:389–400.
- Matsuo S, Imai E, Horio M, Collaborators developing the Japanese equation for estimated GFR. Revised Equations for Estimated GFR From Serum Creatinine in Japan. Am J Kidney Dis. 2009;53:982–992.
- Mittal BV, Alexander MP, Rennke HG, Singh AK. Atheroembolic renal disease: A silent masquerader. Kidney Int. 2008;73:126–130.
- Lye WC, Cheah JS, Sinniah R. Renal cholesterol embolic disease. Case report and review of the literature. Am J Nephrol. 1993;13:489–493.
- Maurizi CP, Barker AE, Truehart RE. Atheromatous emboli: A postmortem study with special reference to the lower extremities. Arch Pathol. 1968;86:528–534.
- Manganoni AM, Venturini M, Scolari F, The importance of skin biopsy in the diverse clinical manifestations of cholesterol embolism. Br J Dermatol. 2004;150:1230–1231.
- Kasinath BS, Lewis EJ. Eosinophilia as a clue to the diagnosis of atheroembolic renal disease. Arch Intern Med. 1987;147:1384–1385.
- Modi KS, Rao VK. Atheroembolic renal disease. J Am Soc Nephrol. 2001;12:1781–1787.
- Hasegawa M, Kawashima S, Shikano M, The evaluation of corticosteroid therapy in conjunction with plasma exchange in the treatment of renal cholesterol embolic disease. Am J Nephrol. 2000;20:263–267.
- Eliot RS, Kanjuh VI, Edwards JE. Atheromatous embolism. Circulation. 1964;30:611–618.
