Abstract
Background: Acute kidney injury (AKI) is a serious complication after coronary artery bypass grafting and is closely associated with high mortality. The objective of the present study was to identify the incidence and risk factors for AKI after off-pump coronary artery bypass grafting (OPCAB) and to construct a risk model for prediction of AKI after OPCAB. Methods: We retrospectively studied 448 adult patients who underwent isolated OPCAB between April 2006 and July 2007. AKI was defined as an increase in serum creatinine of 0.3 mg/dL or 50% within 48 h after surgery. Multivariate analysis was used to evaluate risk factors for AKI after OPCAB and a risk model was developed with a weighted score based on the odds ratio. Results: The incidence of AKI was 7.6% (n = 34). Most patients (97%) had mild AKI. Independent preoperative risk factors of postoperative AKI were identified as high systolic blood pressure, decreased glomerular filtration rate, and coronary angiography (CAG) less than 7 days prior to OPCAB. The incidence of AKI across each increasing score level increased from 2.2% to 60%. Conclusion: AKI after OPCAB was common. High systolic blood pressure, renal dysfunction, and CAG less than 7 days prior to cardiac surgery were associated with AKI after OPCAB. Our risk model may provide information to clinicians and patients about the risk of postoperative AKI.
INTRODUCTION
Postoperative acute kidney injury (AKI) is one of the most serious complications in patients undergoing coronary artery bypass grafting (CABG).Citation1–3 Acute elevations in serum creatinine showed a linear association with increased risk for adverse outcomes after cardiac surgery.Citation4–6
Off-pump coronary artery bypass grafting (OPCAB), which eliminates the need for cardiopulmonary bypass (CPB), has been reported to be associated with a lower risk of AKI and a decrease in the requirement for renal replacement therapy.Citation7,Citation8 However, a mild increase in serum creatinine remains a frequent complication after OPCAB and is associated with increased mortality after cardiac surgery.Citation8,Citation9
Recently, the Acute Kidney Injury Network (AKIN) proposed an alternative classification, which focused on smaller changes in serum creatinine than those considered in the RIFLE (R–renal risk, I–injury, F–failure, L–loss of kidney function, E–end stage renal disease) criteria.Citation10 The diagnostic criteria proposed by AKIN are an abrupt (within 48 h) absolute increase in the serum creatinine concentration of ≥0.3 mg/dL from baseline, a percentage increase in serum creatinine of ≥50%, or oliguria of less than 0.5 mL/kg/h for more than 6 h.Citation11 As no effective therapy for AKI is available at present, there is considerable interest in preventing AKI. Therefore, this study was conducted to evaluate the incidence of and risk factors for AKI, defined by the AKIN criteria, after OPCAB.
METHODS
Between April 2006 and July 2007, 472 consecutive adult (>18 years old) patients who underwent isolated OPCAB were identified using the electronic databases of the Samsung Medical Center, Seoul, Korea. Of these, we excluded patients with incomplete data (n = 14), with a preoperative glomerular filtration rate (GFR) <15 mL/min/1.73 m2 (n = 6), who received redo CABG (n = 2), or who died of causes unrelated to renal injury (n = 2). Data from the remaining 448 patients were analyzed in this study.
The patients' data were extracted from electronic medical records and an electronic database. The demographic and anthropometric data collected were age, sex, and body mass index (weight divided by height squared). The clinical and laboratory data collected were systolic blood pressure (SBP), diastolic blood pressure, diabetes mellitus, history of cerebrovascular accident (previous stroke and/or transient ischemic attack), and/or prior repair (endarterectomy or angioplasty) because of carotid artery obstruction, history of a peripheral arterial occlusive disease (exertional claudication, and/or previous revascularization procedure to legs, and/or radiographic evidence of peripheral arterial obstruction), chronic obstructive pulmonary disease (functional disability, and/or chronic bronchodilator therapy, and/or forced expiratory volume in 1 s/forced vital capacity <70%), recent myocardial infarction (MI) less than 7 days prior to cardiac surgery, left ventricular ejection fraction, use of intra-aortic balloon pump prior to surgery, preoperative use of inotropics, number of coronary arteries ≥50% stenosis, and coronary angiography (CAG) less than 7 days prior to cardiac surgery. Preoperative renal function was estimated by the Cockcroft–Gault equationCitation12 and adjusted for each 1.73 m2 of body surface area.
The clinical outcome measure was the occurrence of AKI after OPCAB. AKI was defined using the AKIN criteria by comparing the highest serum creatinine within 48 h after surgery with preoperative serum creatinine. Patients who met the AKIN criteria for AKI were classified as “AKI”, whereas those who did not were classified as “no AKI”. The severity of AKI was stratified according to the AKIN criteria as follows: stage 1 (increase of serum creatinine ≥50% or ≥0.3 mg/dL), stage 2 (increase of serum creatinine >100%), and stage 3 (increase of serum creatinine >200% or ≥0.5 mg/dL with serum creatinine ≥4.0 mg/dL).Citation11
Statistical Analysis
Continuous variables were expressed as medians with interquartile range or mean ± standard deviation. Categorical variables were displayed as percentages. Univariate and multivariate logistic regression analyses were performed to determine the significant predictors of AKI after OPCAB. Variables with a p-value <0.1 by univariate analysis were entered into a multivariate analysis to identify independent risk factors for AKI. SBP and GFR were categorized; odds ratios and 95% confidence intervals are presented for each category. On multivariate analysis, variables retained at p-value <0.05 were defined as independent risk factors of AKI. Based on the odds ratio, weighted scores were determined for each variable, and incidence of AKI was evaluated according to these risk scores. The contributed area under the curve for each significant variables was calibrated, and then, to assess the power of discrimination of the risk model, the area under the receiver operating characteristics (ROC) curve was measured. All analyses were performed with SPSS version 12.0 (SPSS Inc., Chicago, IL, USA). A p-value of <0.05 was considered significant.
RESULTS
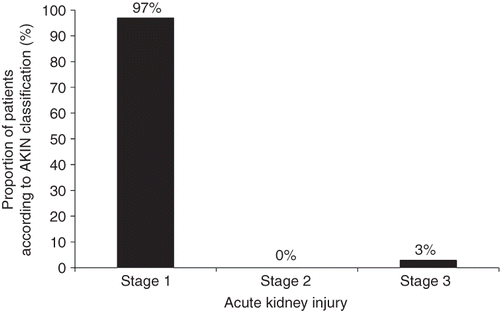
shows the baseline characteristics of the 448 patients. The median age of patients was 65 (59–70) years and 118 (26.3%) patients were female. Preoperative GFR was 72 ± 19 mL/min/1.73 m2. Postoperative AKI occurred in 34 (7.6%) patients (). Most patients who developed AKI fell within the classification of stage 1 ().
Table 1. Baseline characteristics of patients who underwent isolated first off-pump coronary artery bypass graft surgery
Table 2. Univariate analysis of acute kidney injury following isolated first off-pump coronary artery bypass graft surgery
Table 2 displays the significant variables associated on univariate analysis with the development of AKI. There was a linear increase in risk for AKI with decreasing baseline GFR (p < 0.001 for trend). The risk of AKI was 5.1%, 10.6%, 22.7%, and 30% in patients with preoperative GFR ≥ 60, 45–60, 35–45, or 15–30 mL/min/1.73 m2, respectively. Recent MI increased the postoperative AKI (p = 0.042). The risk of AKI increased from 2.6% to 17.9% as SBP rose from <120 to ≥160 mmHg (p = 0.005 for trend).
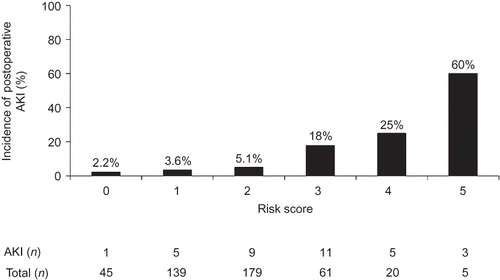
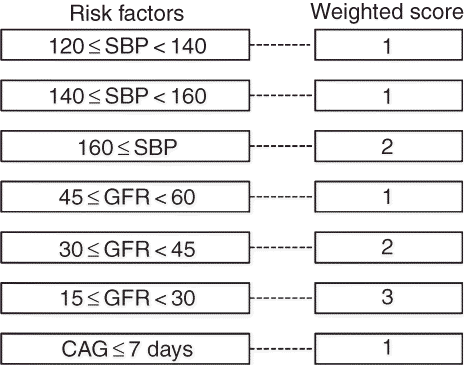
To evaluate the independent contributions of predictors identified by univariate analysis, multivariate analysis was performed. The outcome is described in . Significant predictors for AKI were higher SBP, decreased preoperative GFR, and CAG less than 7 days prior to OPCAB. The weighted scores were graded for each variable, using the odds ratio of the multivariate analysis. The risk model is presented in . The risk of AKI was estimated according to the risk score that ranged from a minimum of 0 to a maximum of 6 (). Patients with a higher risk score had a graded association with a higher incidence of AKI. The proportion of AKI in patients with a score of ≥4 was 32%.
Table 3. Multivariate analysis of acute kidney injury following isolated first off-pump coronary artery bypass graft surgery
Figure 2. A risk-scoring scheme for estimating acute kidney injury after off-pump coronary artery bypass grafting.
Notes: SBP, systemic blood pressure; GFR, glomerular filtration rate; CAG, coronary angiography.
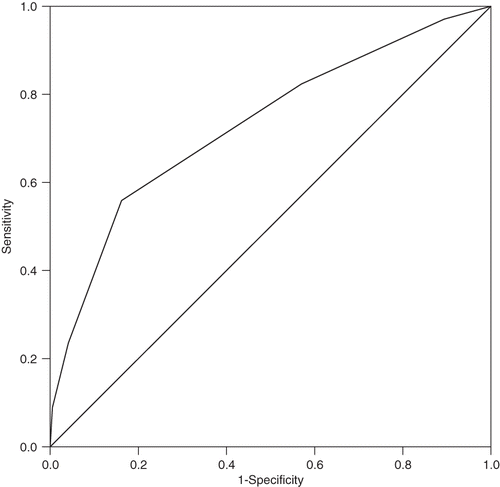
The ROC curve was described in . The area under the curve for score was 0.73 (95% CI 0.64–0.83).
DISCUSSION
This study evaluated the incidence and risk factors for AKI, defined using the AKIN classification, after OPCAB surgery. Of 448 patients, 34 (7.6%) experienced AKI after the first isolated OPCAB. High SBP, preoperative renal dysfunction, and CAG less than 7 days prior to the operation were risk factors for postoperative AKI and the incidence of AKI showed an increasing trend in patients with a higher risk score.
The incidence of AKI after CABG has been reported to range from 5% to 30%, depending on the criteria used to define AKI.Citation13–15 In our study, the incidence of AKI was 7.3% based on the AKIN classification, which is comparable to the 8–14% reported in previous studies that assessed the incidence of AKI after OPCAB using the AKIN criteria.Citation8,Citation16
In most patients with postoperative AKI, the severity of AKI was mild compared with previous published studies. This may arise from differences in our cohort with respect to the preoperative characteristics of patients. Our study excluded CABG with CPB, redo CABG, and valve or aortic surgery, which are known to be risk factors for AKI after cardiac surgery.Citation3,Citation8,Citation13 We excluded patients with severe renal impairment (GFR <15 mL/min/1.73 m2). For our subjects, the mean preoperative GFR was 72 ± 19 mL/min/1.73 m2 and 74% of the patients had a GFR ≥60 mL/min/1.73 m2. Kinoshita et al.Citation17 and Li et al.Citation18 showed that the increment of serum creatinine after cardiac surgery was mild in patients with GFR ≥60 mL/min/1.73 m2. Furthermore, conditions that could contribute to the development of renal ischemia were less common compared with previous studies.Citation8,Citation16 In our study, the number of patients who required intra-aortic balloon pump or inotropics prior to surgery was 0.4% and 1.1%, retrospectively, and 4.1% of patients had a left ventricular ejection fraction of <35%.
Our study analyzed the risk factors for AKI and focused on the preoperative characteristics of patients. One of the major risk factors for the development of AKI after OPCAB is the presence of preoperative renal dysfunction. The risk for AKI after OPCAB was increased approximately 3-fold in patients with GFR ranging from 45 to 60 mL/min/1.73 m2, and up to 12-fold in patients with GFR ranging from 15 to 30 mL/min/1.73 m2. This finding is comparable with the results of previous studies.Citation3,Citation8,Citation13,Citation19 High preoperative SBP may contribute to the development of AKI. The risk of AKI was 2.6%, 9.8%, 8.4%, and 17.9% in patients with SBP <120, 120–140, 140–160, or ≥160 mmHg, retrospectively. In our study, the incidence of AKI was not increased significantly in patients with SBP 140–160 mmHg compared with patients with SBP <120 mmHg (p = 0.054); however, an association between high SBP and AKI was identified in a previous study.Citation13 Exposure to contrast media is a well-known risk factor for development of AKI.Citation20 However, previous studies failed to account for the influence of CAG on AKI after cardiac surgery.Citation3,Citation13,Citation21 Recent limited data identified an adverse effect on postoperative AKI of CAG prior to cardiac surgery.Citation22–24 Two of those studies suggested that CAG within 5 days of cardiac surgery was associated with postoperative AKI.Citation23,Citation24 The risk of AKI was highest when cardiac surgery was performed on the same day as CAG.Citation23,Citation24 Our study showed that CAG less than 7 days prior to OPCAB was associated with a nearly 3-fold increase in the incidence of postoperative AKI.
The ability to predict the development of AKI after cardiac surgery is an important tool for informing patients and clinicians and is useful to improve the quality of patient care. Several studies have reported specific risk models for the development of AKI after cardiac surgery.Citation13,Citation19,Citation21,Citation25,Citation26 Those studies evaluated risk factors for AKI in patients undergoing cardiac surgery with CPB. However, we identified the risk factors, designed a risk model for AKI after OPCAB, and evaluated the risk of postoperative AKI. The area under the ROC curve for AKI score was 0.73; this may allow us to use the risk model in prediction of AKI after OPCAB. However, validation tests are needed to apply the risk model for AKI in other patient groups.
In the AKIN classification, changes in serum creatinine levels and urine output are used to evaluate renal function. We used the change in serum creatinine to define AKI. In practice, there are limitations to assessing the urine output; it may be influenced by hydration status and use of diureticsCitation11 and be maintained in patients with AKI.Citation27 However, it is easy to measure serum creatinine levels in clinical practice and the serum creatinine criterion is more valuable than urine output as a predictor of mortality.Citation28 Thus, prognostic significance and ease of measurement facilitate the use of serum creatinine level rather than urine output.
This study has several limitations. First, it was retrospective in design, which meant that it was unable to select and analyze possible causes of renal dysfunction such as surgical priority and radiocontrast dose. Second, the long-term outcome for patients with AKI was not assessed. However, the association between postoperative AKI and outcomes was established in previous studies.Citation2,Citation3,Citation14 Third, this study was performed using data from a single center, so our findings may not be generalizable to the entire population of patients undergoing OPCAB. Finally, the risk stratification model was not evaluated for general use in other patients undergoing OPCAB. Our model is required to be validated at multiple centers to establish its applicability.
In conclusion, we evaluated the incidence of and risk factors for AKI in patients who underwent OPCAB. Postoperative AKI was relatively common. High SBP, preoperative renal dysfunction, and CAG less than 7 days prior to cardiac surgery were associated with AKI after OPCAB. Using our risk model for AKI, we identified high-risk patients and the incidence of AKI, allowing communication of the risk to the patients, and efficient perioperative care in high-risk patients. However, further investigations are warranted to develop clinical and therapeutic strategies to reduce the incidence of AKI and improve the outcomes of patients after OPCAB.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365–3370.
- Loef BG, Epema AH, Smilde TD, . Immediate postoperative renal function deterioration in cardiac surgical patients predicts in-hospital mortality and long-term survival. J Am Soc Nephrol. 2005;16:195–200.
- Mangano CM, Diamondstone LS, Ramsay JG, Aggarwal A, Herskowitz A, Mangano DT. Renal dysfunction after myocardial revascularization: Risk factors, adverse outcomes, and hospital resource utilization. The Multicenter Study of Perioperative Ischemia Research Group. Ann Intern Med. 1998;128:194–203.
- Hobson CE, Yavas S, Segal MS, . Acute kidney injury is associated with increased long-term mortality after cardiothoracic surgery. Circulation. 2009;119:2444–2453.
- Coca SG, Yusuf B, Shlipak MG, Garg AX, Parikh CR. Long-term risk of mortality and other adverse outcomes after acute kidney injury: A systematic review and meta-analysis. Am J Kidney Dis. 2009;53:961–973.
- Praught ML, Shlipak MG. Are small changes in serum creatinine an important risk factor? Curr Opin Nephrol Hypertens. 2005;14:265–270.
- Nigwekar SU, Kandula P, Hix JK, Thakar CV. Off-pump coronary artery bypass surgery and acute kidney injury: A meta-analysis of randomized and observational studies. Am J Kidney Dis. 2009;54:413–423.
- Massoudy P, Wagner S, Thielmann M, . Coronary artery bypass surgery and acute kidney injury – impact of the off-pump technique. Nephrol Dial Transplant. 2008;23:2853–2860.
- Zhang WF, Gu TX, Diao C, . Comparison of transient changes in renal function between off-pump and on-pump coronary artery bypass grafting. Chin Med J (Engl). 2008;121:1537–1542.
- Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure – definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–R212.
- Mehta RL, Kellum JA, Shah SV, . Acute kidney injury network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31.
- Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41.
- Chertow GM, Lazarus JM, Christiansen CL, . Preoperative renal risk stratification. Circulation. 1997;95:878–884.
- Lassnigg A, Schmidlin D, Mouhieddine M, . Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: A prospective cohort study. J Am Soc Nephrol. 2004;15:1597–1605.
- Provenchere S, Plantefeve G, Hufnagel G, . Renal dysfunction after cardiac surgery with normothermic cardiopulmonary bypass: Incidence, risk factors, and effect on clinical outcome. Anesth Analg. 2003;96:1258–1264.
- Lassnigg A, Schmid ER, Hiesmayr M, . Impact of minimal increases in serum creatinine on outcome in patients after cardiothoracic surgery: Do we have to revise current definitions of acute renal failure? Crit Care Med. 2008;36:1129–1137.
- Kinoshita T, Asai T, Murakami Y, Suzuki T, Kambara A, Matsubayashi K. Preoperative renal dysfunction and mortality after off-pump coronary artery bypass grafting in Japanese. Circ J. 2010;74:1866–1872.
- Li SY, Chen JY, Yang WC, Chuang CL. Acute kidney injury network classification predicts in-hospital and long-term mortality in patients undergoing elective coronary artery bypass grafting surgery. Eur J Cardiothorac Surg. 2011;39:323–328.
- Palomba H, de Castro I, Neto AL, Lage S, Yu L. Acute kidney injury prediction following elective cardiac surgery: AKICS score. Kidney Int. 2007;72:624–631.
- Pannu N, Wiebe N, Tonelli M. Prophylaxis strategies for contrast-induced nephropathy. JAMA. 2006;295:2765–2779.
- Mehta RH, Grab JD, O'Brien SM, . Bedside tool for predicting the risk of postoperative dialysis in patients undergoing cardiac surgery. Circulation. 2006;114:2208–2216.
- Ranucci M, Ballotta A, Kunkl A, . Influence of the timing of cardiac catheterization and the amount of contrast media on acute renal failure after cardiac surgery. Am J Cardiol. 2008;101:1112–1118.
- Del Duca D, Iqbal S, Rahme E, Goldberg P, de Varennes B. Renal failure after cardiac surgery: Timing of cardiac catheterization and other perioperative risk factors. Ann Thorac Surg. 2007;84:1264–1271.
- Medalion B, Cohen H, Assali A, . The effect of cardiac angiography timing, contrast media dose, and preoperative renal function on acute renal failure after coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2010;139:1539–1544.
- Wijeysundera DN, Karkouti K, Dupuis JY, . Derivation and validation of a simplified predictive index for renal replacement therapy after cardiac surgery. JAMA. 2007;297:1801–1809.
- Thakar CV, Arrigain S, Worley S, Yared JP, Paganini EP. A clinical score to predict acute renal failure after cardiac surgery. J Am Soc Nephrol. 2005;16:162–168.
- Diamond JR, Yoburn DC. Nonoliguric acute renal failure. Arch Intern Med. 1982;142:1882–1884.
- Haase M, Bellomo R, Matalanis G, . A comparison of the RIFLE and acute kidney injury network classifications for cardiac surgery-associated acute kidney injury: A prospective cohort study. J Thorac Cardiovasc Surg. 2009;138:1370–1376.