Abstract
Background: This study was carried out to assess the efficacy of intravenous administration of alfacalcidol once weekly versus thrice weekly in patients with poorly controlled secondary hyperparathyroidism. Methods: Thirty-six hemodialysis patients with intact parathyroid hormone (i-PTH) > 31.8 pmol/L were divided into two groups. Eighteen patients (Group 1) were given once weekly alfacalcidol for 6 months. The starting dose was 3 µg, which was increased or decreased by 1 µg per week. Eighteen patients (Group 2) were given thrice weekly alfacalcidol for 6 months. The starting dose was 1 µg, which was increased or decreased by 0.5 µg per dose. The dose was increased or decreased according to serum-corrected calcium (CCa), phosphorus (P), and i-PTH. Serum-CCa and P were measured weekly, whereas serum i-PTH and alkaline phosphatase were determined every month. Results: Intact-PTH reduced significantly ( p < 0.001) from 86 ± 33.20 pmol/L to 31.04 ± 7.77 pmol/L and from 83.64 ± 32.12 pmol/L to 33.09 ± 11.37 pmol/L post-treatment in Groups 1 and 2, respectively. Fifty-six percent of the patients had i-PTH ≤ 31.8 pmol/L at the last observation. Serum alkaline phosphatase reduced significantly ( p < 0.001) from 227.94 ± 129.86 IU/L to 163.17 ± 95.29 IU/L and from 285.39 ± 232.36 IU/L to 202.56 ± 165.84 IU/L post-treatment in Groups 1 and 2, respectively. There were no significant differences in serum levels of CCa, P, or their product. Conclusion: Intravenous alfacalcidol thrice or once weekly is safe and effectively reduced the levels of i-PTH in hemodialysis patients.
INTRODUCTION
Secondary hyperparathyroidism (SHPT) is the most frequent pattern of renal osteodystrophy and is caused by several interrelated factors, such as hypocalcemia, hyperphosphatemia, and calcitriol deficiency.Citation1 Many disturbances, such as changes in parathyroid hormone (PTH) and vitamin D (VD) metabolism, a skeletal resistance to calcemic PTH action, altered calcium–PTH dynamic relationship, and decreased levels of VD receptor and calcium sensor receptor have also been implicated in the pathogenesis of SHPT.Citation1,Citation2 Excessive PTH levels alter the bone remodeling process by increasing bone formation and resorption, in association with various degrees of medullar fibrosis. Control of SHPT is one of the main objectives in the management of uremic bone disease.Citation3 According to Kidney Disease Outcomes Quality Initiative (K/DOQI) guidelines, in patients with stage 5 chronic kidney disease, the serum-corrected calcium (CCa) level should be 2.1–2.38 mmol/L, serum phosphorus should be 1.13–1.78 mmol/L, calcium–phosphorus (Ca × P) product should be <4.44 mmol2/L2, and the intact parathyroid hormone (i-PTH) level should be 15.9–31.8 pmol/L. Meeting these targets is not easy to achieve in all patients.Citation4,Citation5 Usually, the PTH level is controlled with oral VD preparations in the early stage. When PTH control is difficult, this therapy is switched to intravenous (IV) VD therapy.Citation6 Alfacalcidol (1-α-OH-VD) has been demonstrated to be a good metabolite with the capacity to downregulate parathyroid gland activity, with scant hypercalcemic potential when used at doses adjusted to the degree of SHPT in hemodialysis (HD) patients.Citation7–9 Nevertheless, the difficulties in controlling the disease in clinical practice constitute a problem for adhering to the treatment recommendations. Based on this fact, we conducted an observational, prospective study in chronic HD patients with high i-PTH levels to evaluate the efficacy and safety of IV administration of a high dose of alfacalcidol once weekly versus thrice weekly.
PATIENTS AND METHODS
Patient Selection
A total of 36 patients of either sex and over 18 years of age, undergoing three times weekly HD for >6 months and with a diagnosis of SHPT (PTH > 31.8 pmol/L), for which alfacalcidol treatment was planned, were recruited from August 2009 to May 2010. All patients were refractory to an oral pulse of 3 µg alfacalcidol (one-alpha, Leo Pharmaceutical Products Sarath, Athens, Greece) twice or thrice weekly. Patients were considered refractory to oral therapy if they do not respond to treatment, develop hypercalcemia and/or hyperphosphatemia. Patients with a serum-CCa > 2.61 mmol/L, serum-P 2.098 mmol/L, or Ca × P product > 5.649 were excluded. Subjects were only included in the study after signing the informed written consent, and the Institutional Review Board (IRB)/Ethics Committee approval was obtained and the study adhered to the principles of the Declaration of Helsinki.
Study Protocol
The study protocol consisted of an initial 4-week washout period followed by a treatment period lasting up to 6 months. Eligible patients were assigned to two groups. Eighteen patients (Group 1) were given IV alfacalcidol once weekly at the end of their mid-week dialysis session for 6 months. Eighteen patients (Group 2) were given IV alfacalcidol thrice weekly at the end of their dialysis session for 6 months. Alfacalcidol was started at an initial dose of 3 µg/week and 1 µg/dose in Groups 1 and 2, respectively, and the dose was increased or decreased by 1 µg/week and 0.5 µg/dose in Groups 1 and 2, respectively, according to serum-CCa and P and i-PTH. The drug was temporarily withheld for a week upon the development of hypercalcemia (2.61 mmol/L), hyperphosphatemia (>1.78 mmol/L), or a persistent Ca × P (>4.44 mmol2/L2). The drug was resumed at a dosage reduced by one step with the adjustment of phosphate binders. All patients were receiving calcium carbonate and/or sevelamer tablets as phosphate binder before starting the study. All patients received their dialysis treatment three times per week, 4 h per session. Polysulfone dialyzers and a bicarbonate bath with a dialysate Ca concentration of 1.25 mmol/L and Na concentration of 136 mmol/L were used for dialysis. The blood flow rate was ≥250 mL/min and the dialysate flow rate was 800 mL/min. All patients were receiving recombinant darbepoetin intravenously once weekly or every 2 weeks post dialysis.
Biochemical Parameters
During the study period, serum-CCa and P were measured weekly. Serum i-PTH and alkaline phosphatase (ALP) were determined monthly. Intact-PTH was measured with the Coat-A-Count immunoradiometric assay method (Diagnostic Products Corporation, Los Angeles, CA, USA).
STATISTICAL ANALYSIS
The Statistical Package for Social Sciences version 16.0 (SPSS, Chicago, IL, USA) was used for data processing; and the chi-square test, paired t-test, two t-test, and the nonparametric Mann–Whitney U-test were used to find the study association between variables, as appropriate. All data were summarized by means of the appropriate descriptive statistics, and the level of significance for all tests was established at the 0.05 level (two sided).
RESULTS
The Demographic Characteristics
As shown in both groups were similar with regard to age, gender, and HD duration.
Table 1. Demographic characteristics of the studied patients
Biochemical Parameters
As shown in , there were no significant changes in the serum-CCa and P levels and Ca × P product post-treatment compared with the baseline in Group 1. Comparison of Group 1 and Group 2 did not show significant differences during the study period.
Table 2. Biochemical parameters for the studied patients
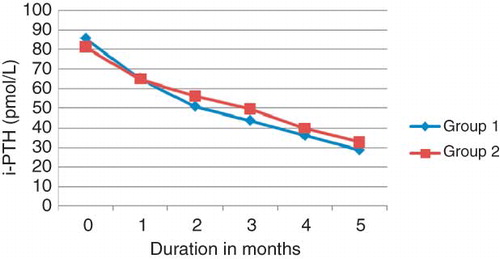
As shown in and , the mean baseline i-PTH was 86 ± 33.20 pmol/L and 83.64 ± 32.12 pmol/L in Groups 1 and 2, respectively. The reduction of i-PTH to 31.04 ± 7.77 pmol/L and 33.09 ± 11.33 pmol/L at the end of study period in Groups 1 and 2, respectively, was highly significant (p < 0.001) in comparison to the baseline. Comparing the mean i-PTH of both groups at the beginning of the study and during the treatment period was not significant. About 61% and 50% of the patients had i-PTH ≤ 31.8 pmol/L in Groups 1 and 2, respectively, at the last observation.
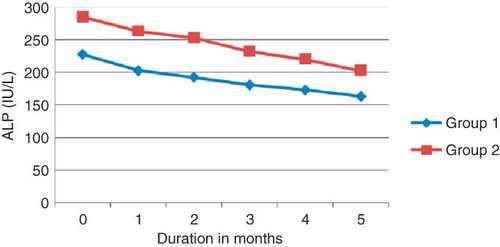
shows the response of ALP to IV alfacalcidol in both groups. The mean baseline ALP was 227.94 ± 129.86 IU/L and 285.39 ± 232.36 IU/L in Groups 1 and 2, respectively. The reduction of ALP to 163.17 ± 95.29 IU/L and 202.56 ± 165.84 IU/L at the end of the treatment period in Groups 1 and 2, respectively, was highly significant (p < 0.001) compared with the baseline. Comparing the mean ALP of both groups at the beginning of the study and during the treatment period was not significant.
Drug Utilization
Alfacalcidol was temporarily withheld for 1 week in two patients in Group 1 and one patient in Group 2 because of marginal rise in the Ca × P product due to hyperphosphatemia after 1 month of treatment. At the end of the study, the mean dose of calcium carbonate and sevelamer remained almost unchanged through the study period. There were no significant differences in the mean doses of calcium carbonate (2.27 ± 0.999 vs. 1.8 ± 0.713 gm/day) and sevelamer (3.31 ± 1.69 vs. 3.37 ± 1.83 gm/day) administration between group 1 & 2, respectively at the end of the study, (P > 0.05).
DISCUSSION
SHPT is one of the most serious complications of patients on long-term HD.Citation10,Citation11 Although, early initiation of prophylactic measures and calcitriol pulse therapy Citation12 in cases with advanced hyperparathyroidism have had a positive impact on patient management, there are still difficulties to select the most appropriate therapy for the individual patient with advanced SHPT.Citation13,Citation14 Clinical trials suggest that attaining pharmacological levels of serum 1,25(OH)2 vitamin D3 through IV route is more effective in suppressing serum PTH levels than oral therapy.Citation15,Citation16 Other studies also have shown that IV route is associated with fewer episodes of hypercalcemia than the oral administered route.Citation17,Citation18 IV alfacalcidol administration bypasses intestinal degradation of the hormone and thereby achieves a concentration of the active VD compound in the parathyroid glands where it causes a direct inhibition of PTH synthesis through action on the specific VD receptors before any significant rise in serum Ca or P.Citation19
In this study, IV alfacalcidol pulse therapy reduced i-PTH by 55 ± 25.43 pmol/L and 50.55 ± 20.79 pmol/L after 6 months of therapy in Groups 1and 2, respectively, this representing a median reduction of 60% from baseline. The cumulative experience indicates that this is considered a successful response to alfacalcidol.Citation20 On the other contrary, 56% of the alfacalcidol-treated patients had a final i-PTH level of ≤31.8 pmol/L, this value generally being considered to reflect adequate control of SHPT. Moreover, there is a highly significant reduction on the serum ALP levels after 6 months of treatment in both groups.
The results of this study have also shown that the use of IV alfacalcidol pulse therapy once weekly is a safe and effective treatment for advanced SHPT. Even though there was no significant difference in i-PTH and other parameters in both groups, some aspects however are marginally different in both routes of alfacalcidol administration. The concomitant marginal increases in Ca and P concentrations were not significant in either group. Furthermore, the Ca × P product and serum-CCa and P remained close to the target levels recommended by the K/DOQI guideline.Citation21 The consequences of an elevated Ca × P product are well recognized to involve vascular and soft tissue calcification and tumor calcinosis.Citation22–24 High levels of P, both at baseline and over the follow-up period, are associated with mortality in dialysis patients. High levels of the Ca × P product and i-PTH are also associated with high mortality.Citation25 González et al. stated that alfacalcidol when used according to the recommendations reduces serum i-PTH, albeit with considerable variations and at the expense of a slight increase in serum calcium and phosphate levels. When alfacalcidol is used in patients with mild or moderate hyperparathyroidism, the doses needed are lower than in patients with severe hyperparathyroidism.Citation26 Also Taniguchi et al. reported that IV VD therapy in the phase of diffuse hyperplasia may prevent the deterioration to nodular hyperplasia, avoiding parathyroidectomy. In addition, good control of the serum levels of PTH, Ca, and P may inhibit vascular calcification, improving the prognosis.Citation6 Shoji et al.Citation27 showed in a small observational study that patients taking alfacalcidol had reduced risk of cardiovascular disease (CVD) death compared to patients who were not on VD. Tentori et al.Citation28 published similar findings in a larger cohort treated with vitamin D receptor activator (VDRA). Another study showed reduced mortality even in incident dialysis patients treated with VDRA.Citation25 Furthermore, observational studies support that the newer VDRAs (paricalcitol and doxercalciferol) have better outcomes than traditional therapy with calcitriol.Citation29 This may be due to the tendency of calcitriol to cause hypercalcemia and hyperphosphatemia. Alternatively, VDRAs may act on different biologic targets.Citation25,Citation30 In contrast, a meta-analysis by Palmer et al.Citation31 found no beneficial effects on mortality or vascular calcification in patients with chronic kidney disease who had received VDRA. The newly developed, non-aluminum, non-calcium-based phosphate binders, such as sevelamer carbonate,Citation32 lanthanum carbonate,Citation33 and calcimimetic agentsCitation34 represent a breakthrough in the management of hyperphosphatemia and SHPT. Our study combined this new dosage regimen of alfacalcidol with sevelamer and diet restriction in hyperphosphatemic patients to offer means to achieve the target i-PTH level. Notably, in our study patients receiving once weekly therapy required lower dosages of alfacalcidol to achieve the target i-PTH than those on thrice weekly therapy. This finding is consistent with the current recommendation to the minimal therapeutic dose of VD. Furthermore, it will minimize the short- and long-term effects of therapy.
CONCLUSION
IV alfacalcidol administration once weekly is a safe and effective therapeutic intervention that suppresses PTH secretion in patients with severe SHPT. A similar safety margin was observed with once and thrice weekly regimens of alfacalcidol treatment as regards hypercalcemia and hyperphosphatemia.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Goodman WG. Recent developments in the management of secondary hyperparathyroidism. Kidney Int. 2001;59:1187–1201.
- Drüeke TB. Parathyroid gland hyperplasia in uremia. Kidney Int. 2001;59:1182–1183.
- Coburn JW, Llach F. Renal osteodystrophy. In: Narins RG, ed. Clinical Disorders of Fluid and Electrolyte Metabolism. 5th ed, New York, NY: McGraw Hill Book Co.; 1994:1299–1377.
- Salem MM. Hyperparathyroidism in the hemodialysis population: A survey of 612 patients. Am J Kidney Dis. 1997;29:862–865.
- Al Aly Z, Gonzalez EA, Martin KJ, Gellens ME. Achieving K/DOQI laboratory target values for bone and mineral metabolism: An uphill battle. Am J Nephrol. 2004;24:422–426.
- Taniguchi M, Tokumoto M, Tsuruya K, . Intravenous calcitriol therapy in an early stage prevents parathyroid gland growth. Nephrol Dial Transplant. 2008;23:3662–3669.
- Fournier A, Morinière Ph, Oprisiu R, . 1-Alpha-hydroxyvitamin D3 derivatives in the treatment of renal bone diseases: Justification and optimal modalities of administration. Nephron. 1995;71:254–283.
- Brandi L, Daugaard H, Egsmose C, . Intermittent intravenous followed by intermittent oral 1 alpha (OH)D3 treatment of secondary hyperparathyroidism in uraemia. J Intern Med. 1996;239:353–360.
- Brandi L, Daugaard H, Nielsen PK, . Long-term effects of intravenous 1 alpha (OH) D3 combined with CaCO3 and low-calcium dialysis on secondary hyperparathyroidism and biochemical bone markers in patients on chronic hemodialysis. Nephron. 1996;74:89–103.
- Statopolsky EA, Burke SK, Dillon MA. Renagel a non absorbed calcium and aluminum free phosphorus binder lowers serum phosphorus and parathyroid hormone. The Renagel Study Group. Kidney Int. 1999;55:299–307.
- Fukagawa M, Kazarna H, Shigematsu T. Management of patients with advanced secondary hyperparathyroidism: The Japanese approach. Nephrol Dial Transplant. 2002;17:1553–1557.
- Akizawa T, Fukagawa M, Koshikawa S, Kurokawa K. Recent progress in management of secondary hyperparathyroidism of chronic renal failure. Curr Opin Nephrol Hypertens. 1993; 2:558–565.
- Gallieni M, Brancaccio D. Which is the preferred treatment of advanced hyperparathyroidism in a renal patient? I. Medical intervention is the primary option in the treatment of advanced hyperparathyroidism in chronic renal failure. Nephrol Dial Transplant. 1994;9:1816–1819.
- Ritz E. Which is the preferred treatment of advanced hyperparathyroidism in a renal patient? II. Early parathyroidectomy should be considered as the first choice. Nephrol Dial Transplant. 1994;9:1819–1821.
- Pitts TO, Piraino BH, Mitro R. Hyperparathyroidism and 1,25 dihydroxyvitamin D in mild, moderate and severe renal failure. J Clin Endocrinol Metab. 1988;67:876–881.
- Andress DL. Intravenous versus oral vitamin D therapy in dialysis patients: What is the question? Am J Kidney Dis. 2001;38:s41.
- Gallieni M, Brancaccio D, Padovese P, . Low dose intravenous calcitriol treatment of secondary hyperparathyroidism in hemodialysis patients. Kidney Int. 1992;42:1191–1198.
- Morinier PH, El Esper N, Viron B, . Improvement of severe secondary hyperparathyroidism in dialysis patients by intravenous 1a-(OH)-D3, oral CaCO3 and low dialysate calcium. Kidney Int. 1993;43(Suppl. 41):121–124
- National Kidney Foundation. K/DOQI clinical practice guide lines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42:S1–S202.
- Cannata JB. Indications and limitations of intravenous calcitriol. Nefrologia. 1995;55:307–314.
- Angelis M, Wong LL, Myers SA, Wong LM. Calciphylaxis in patients on hemodialysis: A prevalence study. Surgery. 1997;122:1083–1090.
- Guerin AP, London GM, Marchais SJ, Metivier F. Arterial stiffening and vascular calcifications in end-stage renal disease. Nephrol Dial Transplant. 2000;15:1014–1021.
- Goodman WG, Goldin J, Kuizon BD, . Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med. 2000;342:1478–1483.
- London GM, Guerin AP, Marchais SL, . Arterial media calcification in end-stage renal disease: Impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant. 2003;18:1731–1740.
- Melamed ML, Eustace LA, Plantinga L, . Changes in serum calcium, phosphorus, and PTH and the risk of death in incident dialysis patients: A longitudinal study. Kidney Int. 2006;70:351–357.
- González MT, Torregrosa JV, Colomé E, . Efficacy of Intravenous alfacalcidol in the treatment of secondary hyperparathyroidism in patients on hemodialysis. Nephron Clin Pract. 2008;108:c141–c147.
- Shoji T, Shinohara K, Kimoto E, . Lower risk for cardiovascular mortality in oral 1alpha-hydroxyvitamin D3 users in a hemodialysis population. Nephrol Dial Transplant. 2004; 19:179–184.
- Tentori F, Hunt WC, Stidley CA, . Mortality risk among hemodialysis patients receiving different vitamin D analogs. Kidney Int. 2006;70:1858–1865.
- Martin KJ, Gonzalez EA. Vitamin D analogs: Actions and role in the treatment of secondary hyperparathyroidism. Semin Nephrol. 2004;24:456–459.
- Teng M, Wolf M, Ofsthun MN, . Activated injectable vitamin D and hemodialysis survival: A historical cohort study. J Am Soc Nephrol. 2005;16:1115–1125.
- Palmer SC, McGregor DO, Macaskill P, . Meta-analysis: Vitamin D compounds in chronic kidney disease. Ann Intern Med. 2007;147:840–853.
- Chertow GM, Burke SK, Dillon MA, Slatopolsky E. Long term effects of sevelamer hydrochloride on the calcium × phosphorus product and lipid profile of hemodialysis patients. Nephrol Dial Transplant. 1999;14:2907–2914.
- Behets GL, Verberckmoes SC, D'Haese PC, De Broe ME. Lanthanum carbonate: A new phosphorus binder. Curr Opin Nephrol Hypertens. 2004;13:403–409.
- Block GA, Martin KL, de Francisco ALM, . Cinacalcet for secondary hyperparathyroidism in patients receiving hemodialysis. N Engl J Med. 2004;350:1516–1525.