Abstract
Although acute renal failure developing due to malignancies is a frequent condition, malignant renal infiltration is rarely observed among these causes. Among all malignant diseases, the hematolymphoid malignancies are the most prone to renal infiltration. Other types involved in cases with lymphoma are glomerulopathies, including immune-complex glomerular diseases such as minimal change disease, membranous glomerulonephritis, membranoproliferative glomerulonephritis, and focal segmental glomerulosclerosis. We present herein the rare case of a 22-year-old male with both membranous glomerulonephritis and CD20 (+) lymphoid infiltration related to Hodgkin's lymphoma in the renal interstitial tissue, as detected on biopsy. The patient was treated with adriamycin, bleomycin, vinblastine, and dacarbazine protocol after pulse corticosteroid treatment, and a dramatic improvement in renal function was observed after 2 days of treatment. In this article, an exceptional renal involvement of Hodgkin's lymphoma is discussed in light of the related literature.
INTRODUCTION
During the course of hematolymphoid malignancies and their treatment process, the kidneys are frequently affected, and the malignancies can even be seen as a cause of severe renal function impairment. In autopsies, the renal parenchymal involvement rate, increasing in direct proportion to disease stage in patients with lymphoma, was reported between 6% and 60%.Citation1–8 In hematolymphoid malignancies, acute renal failure (ARF) can develop as a result of dehydration, hypocalcemia, urethral obstruction, renal vascular compression, paraproteinemia, cryoglobulinemia, amyloidosis, hemolysis, and glomerulonephropathy, or due to causes secondary to treatment, such as tumor lysis syndrome, radiation nephritis, and toxic influences of chemotherapy.Citation1–12
Furthermore, primary renal lymphoma, accounting for 0.1–0.7% of extranodal lymphomas, can also be a very rare cause of ARF.Citation13–19 To date, approximately 150 glomerulonephropathy cases have been reported among all cases with Hodgkin's lymphoma (HL) and non-Hodgkin's lymphoma (non-HL), and 6.8% of them were reported as membranous glomerulonephritis (MGN). This rate is about 5% in HL cases, and only 5 MGN cases have been reported to date. However, the encountered lesion is minimal change disease in approximately half of the cases ().Citation20
Table 1. Incidence and types of glomerulonephropathy associated with lymphoma.Citation20
In this report, a case with ARF developing secondary to HL is discussed. This patient had MGN findings as well as malignant renal infiltration related to HL. To the best of our knowledge, no previous case has been described in the literature with concomitant MGN and CD20 (+) lymphoid infiltration in the interstitial tissue related to HL.
CASE REPORT
A 22-year-old male patient was referred to our emergency department with complaints of nausea, fever, weight loss of 5 kg in 1 month, and decreased urine volume. On physical examination, blood pressure was 110/60 mmHg, pulse rate was 77/min, and body temperature was 37.5°C. He was cachectic in appearance, with squamous–erythematous papular lesions on his skin and axillary and inguinal micro-lymphadenopathies. Referral laboratory findings were as follows: blood urea nitrogen: 106 mg/dL, creatinine: 10.5 mg/dL, lactate dehydrogenase: 259 U/L, sodium: 146 mEq/L, potassium: 5.8 mEq/L, calcium: 9.6 mg/dL, phosphorus: 6.2 mg/dL, uric acid: 3.5 mg/dL, albumin: 3.0 g/dL, sedimentation rate: 106 mm/h and human C-reactive protein: 188 mg/L. Complete urine investigation revealed pH: 7, density: 1007, glucose: negative, and trace amount of protein; on microscopy, there were 10 erythrocytes and 25 leukocytes. There was no growth in urine culture. 24-h urine Esbach revealed 0 g/day, and C3 and C4 were within normal limits. Serology tests for antinuclear antibodies and antineutrophil cytoplasmic antibodies (pANCA and cANCA) were negative. Microbiological findings were as follows: cytomegalovirus, hepatitis B virus, hepatitis C virus, and antihuman immunodeficiency virus were negative; Epstein–Barr antibodies IgM: negative, IgG: positive; parvovirus B19 antibodies IgG and IgM: negative; mumps antibodies IgG and IgM: negative; measles antibodies IgM: negative, measles antibodies IgG: positive; toxoplasma antibodies IgG and IgM: negative; and rubella antibodies IgM: negative, rubella antibodies IgG: positive. Tuberculin skin test was negative, and alpha-fetoprotein, carcinoembryonic antigen (CEA), CA 19-9, and CA 15-3 tumor markers were detected as within normal ranges. Hematological values included the following: WBC: 12,000/mm3, hematocrit: 35%, and platelets: 282,000/mm3. On bone marrow biopsy, cellular density was normal; myelocytic and megakaryocytic lines were normal; eosinophils were slightly increased; and iron was estimated to be (+) 4. Other laboratory findings including serum immunoelectrophoresis were found to be normal.
Renal ultrasonography determined the size and parenchyma gauges of the kidneys to be normal, whereas their echogenicities were increased to grade 3. On the unenhanced thoracoabdominal tomography, multiple lymphadenopathies were seen in the para-aortic and paracaval areas, the largest of which was 27 mm in diameter, and a few lymphadenopathies (largest diameter, 15 mm) were present in the bilateral axillary area.
On the renal biopsy applied in conjunction with ultrasonography, the findings were as follows: light microscopic examination revealed focal tubular atrophy, interstitial fibrosis, and mononuclear cellular infiltration of medium density consisting of small lymphocytes in the interstitium and thickening of the glomerular basement membrane. Immunofluorescence microscopic examination revealed dense granular staining for IgG and C3 in the glomerular basement membrane and focal granular staining for IgM in the basement membrane; however, there was no staining for IgA, fibrinogen, or C1q. On immunohistochemical (IHC) staining, while HL-CD20 (+) B lymphocytes were detected among lymphocytes in the interstitium, CD3 and CD5 (+) small T lymphocytes were observed more dominantly (Figures
Figure 1. Glomerular basement membrane thickening (yellow arrows) and mononuclear cell infiltration (arrows) in kidney interstitium (HE, ×200). HE, Hematoxylin eosinophil.
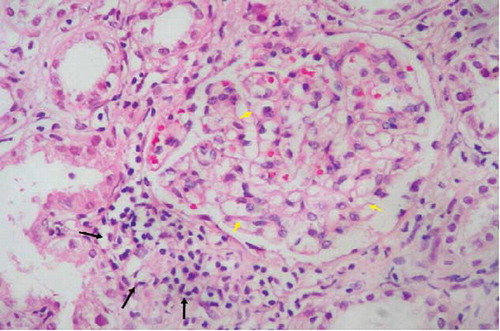
Figure 3. B-cell lymphocytes stained by antibodies CD20 infiltration (arrows) in kidney interstitium (ABP, CD20, ×200). ABP, avidin biotin peroxidase method.
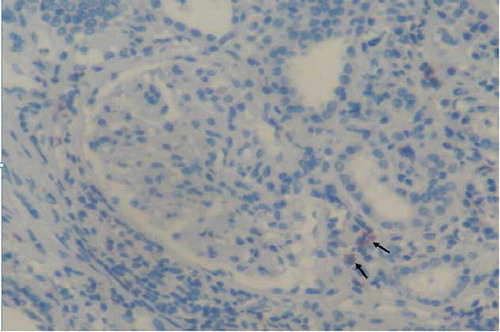
Figure 2. Small T-cell lymphocytes stained by antibodies CD3 infiltration (arrows) in kidney interstitium (ABP, CD3, ×200). ABP, avidin biotin peroxidase method.
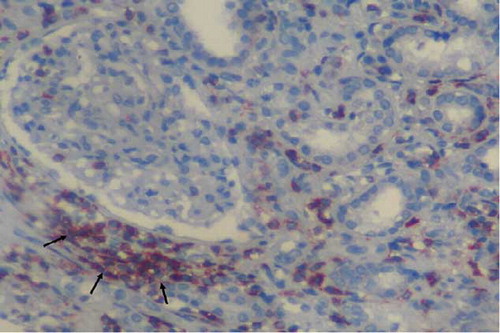
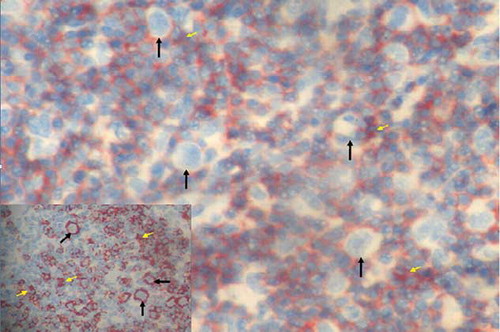
Figure 4. Reed–Sternberg cells and reactive small lymphocytes (arrows) in lymph node sections (HE, ×200). Inset: cytoplasmic CD30 positivity in Reed–Sternberg cells (ABP, CD30, ×400). ABP, avidin biotin peroxidase method. Hematoxylin eosinophil.
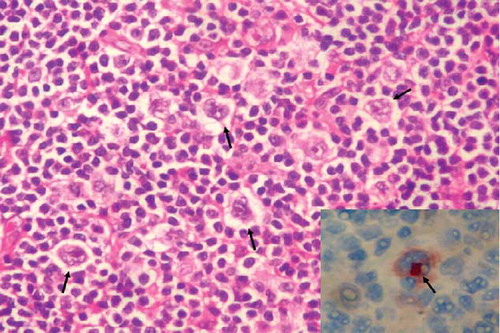
Figure 5. Reed–Sternberg cells (white arrows) and CD3-positive reactive small lymphocytes (yellow arrows) in lymph node sections (ABP, CD3, ×200). Inset: CD20-positive Reed–Sternberg cells (arrows) and CD20-positive reactive small B lymphocytes (yellow arrows) in lymph node sections (ABP, CD20, ×200). ABP, avidin biotin peroxidase method.
Adriamycin (25 mg/m2 on 1st and 15th days), bleomycin (10 mg/m2 on 1st and 15th days), vinblastine (6 mg/m2 on 1st and 15th days), and dacarbazine (375 mg/m2 on 1st and 15th days) (ABVD) protocol was administered to the patient after pulse corticosteroid treatment (1 g/h on each of 3 successive days). ABVD protocol was ended after 8 sessions per cycle of 28 days.
On the second day of treatment, blood urea nitrogen and creatinine values had returned to normal and his dialysis was removed. During the HL treatment period, renal functions were still normal at the 12th-month follow-up.
DISCUSSION
ARF is a frequently encountered condition during the course of malignant diseases. In malignant diseases, causes of ARF include renal vascular compression, dehydration, nephrotoxic or ischemic acute tubular necrosis, hypercalcemia, urinary tract obstruction, paraproteinemia or glomerulonephropathy (especially MGN), or malignant cellular infiltration to the renal parenchyma, which is very rarely seen (10). While renal involvement is especially MGN in cases with solid tumor, minimal change disease is a frequent lesion in HL (20).
In our case, interstitial CD20 (+) lymphoid cellular infiltration, accompanying MGN secondary to HL, is the matter in question. In the literature, no other case has been described to date with these involvements present simultaneously. However, this may be because IHC assessment of biopsy samples was not done in the other patients.
The mechanism of ARF in lymphoid infiltration is not known clearly; however, it may be due to intrarenal obstruction and ischemia as a result of the diffuse infiltration that compresses the renal tubes and microvascular structure.Citation1–8 This assumption is also confirmed by the fact that treatment decreasing tumor load results in rapid improvement in renal functions. Another hypothesis suggests that ARF results from tubular injury and interstitial fibrosis caused by cytokines such as interleukin-1 and 6 and tumor necrosis factor-α and β released from lymphoma cells.Citation21.
In the kidneys, clinically significant malignant infiltration is very rare. However, malignant renal infiltration should be always considered in patients admitted with globally enlarged kidneys and ARF. In this respect, if malignancy is present, biopsy is critical in terms of identifying the renal involvement and its distinguishing features. Probable causes of renal failure can thus be excluded. Furthermore, IHC staining should be performed as well for precisely determining the involvement characteristic in cases with lymphoma. This facilitates probable detection of RS cells and malignant infiltration, which could be overlooked with hematoxylin–eosin staining, in all cases. In addition, the exact nature of the infiltration can be described if IHC staining along with light microscopic examination in cases with infiltrative renal involvement is performed; thus, it can guide the treatment and also predict the response to the treatment. B lymphocytes on the ground and RS cells phenotypically stain positive for CD15, CD30, and CD20 in HL. These staining properties are very important for distinction of HL, especially for lymphocyte-predominant classic HL and nodular-predominant HL as well as for other rare lymphoma types (T-cell/histiocyte-predominant B-cell lymphoma, anaplastic T/null-anaplastic large cell lymphoma or diffuse large B-cell lymphoma).Citation22
Pulse steroid treatment was started while awaiting the results of the renal and inguinal lymph node biopsies, considering the likelihood of a rapidly progressive crescentic glomerulonephritis and the clinical course of the patient. ABVD protocol treatment was started 2 days after completion of pulse steroid treatment upon receipt of the biopsy reports. We believe that the rapid and dramatic response in terms of renal function recovery may be attributed to the lympholytic effect of the corticosteroids as well as their effect on the glomerulonephritis.
In conclusion, we believe that our case is unique because concomitant MGN and CD20 (+) lymphoid infiltration related to HL have not been reported until now. Furthermore, we suggest that IHC evaluation in addition to renal biopsy, light microscopic examination, and immunofluorescence would be useful in patients admitted with ARF in terms of excluding other causes of involvement and identifying the exact nature of the renal involvement.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Sellin L, Friedl C, Klein G, Waldherr R, Rump LC, Weiner SM. Acute renal failure due to a malignant lymphoma infiltration uncovered by renal biopsy. Nephrol Dial Transplant. 2004;19:2657–2660.
- Obrador GT, Price B, O'Meara Y, Salant DJ. Acute renal failure due to lymphomatous infiltration of the kidneys. J Am Soc Nephrol. 1996;8:1348–1354.
- Glicklich D, Sung MW, Frey M. Renal failure due to lymphomatous infiltration of the kidneys. Cancer. 1986;58: 748–753.
- Geffen DB, Fisher RI, Longo DL, Young RC, DeVita VT, Jr. Renal involvement in diffuse aggressive lymphomas: Results of treatment with combination chemotherapy. J Clin Oncol. 1985;3:646–653.
- Leung AC, McKean M, Mactier R, Dobbie JW. Acute oliguric renal failure secondary to lymphomatous infiltration of the kidneys. Postgrad Med J. 1984;60:556–558.
- Malbrain ML, Lambrecht GL, Daelemans R, Lins RL, Hermans P, Zachee P. Acute renal failure due to bilateral lymphomatous infiltrates. Primary extranodal non–Hodgkin's lymphoma (p-ENNHL) of the kidneys: Does it really exist? Clin Nephrol. 1994;42:163–169.
- Bertoncelli C, Airoldi G, Zigrossi P, Catania E, Ramponi A, Monteverde A. Parenchymal renal involvement in three cases of non–Hodgkin's lymphomas: Clinical and pathological features. Haematologica. 1993;78:58–60.
- Lommatzsch S, Bellizzi A, Cathro H, Rosner M. Acute renal failure caused by renal infiltration by hematolymphoid malignancy. Ann Diag Path. 2006;10(4):230–234.
- Feldman L, Basok A, Kachko L, Tovbin D. Lymphomatous infiltration of the kidney associated with glomerulopathy presenting as acute renal failure. Isr Med Assoc J. 2001;3: 541–543.
- Kapoor M, Chan GZ. Malignancy and renal disease. Crit Care Clin. 2001;17:571–598.
- Rault R, Holley JL, Banner BF, el-Shahawy M. Glomerulonephritis and non-Hodgkin's lymphoma: A report of two cases and review of the literature. Am J Kidney Dis. 1992;20:84–89.
- Herrera GA, Joseph L, Gu X, Hough A, Barlogie B. Renal pathologic spectrum in an autopsy series of patients with plasma cell dyscrasia. Arch Pathol Lab Med. 2004;128: 875–879.
- Yasunaga Y, Hoshida Y, Hashimoto M, Miki T, Okuyoma A, Aozasa K. Malignant lymphoma of the kidney. J Surg Oncol. 1997;64:207–211.
- Choi JH, Choi GB, Shim KN, Sung SH, Han WS, Baek SY. Bilateral primary renal non-Hodgkin's lymphoma presenting with acute renal failure. Successful treatment with systemic chemotherapy. Acta Hematol. 1997;97:231–235.
- Ahmad AH, MacLennan GT, Listinsky C. Primary renal lymphoma: A rare neoplasm that may present as a primary renalmass. J Urol. 2005;173(1):239.
- van Gelder T, Michiels JJ, Mulder AH, Klooswijk AIJ, Schalekamp MADH. Renal insufficiency due to bilateral primary renal lymphoma. Nephron. 1992;60(1):108–110.
- Stallone G, Infante B, Manno C, Campobasso N, Pannarale G, Schena FP. Primary renal lymphoma does exist: Case report and review of the literature. J Nephrol. 2000;13: 367–372.
- Brouland JP, Meeus F, Rossert J, Primary bilateral B-cell renal lymphoma: A case report and review of the literature. Am J Kidney Dis. 1994;24:586–589.
- Aozasa K, Tsujımoto M, Sakurai M, Non Hodgkin's lymphoma in Osaka. Jpn Eur J Cancer Clin Oncol. 1985;21:487–492.
- Mallouk A, Pham PT, Pham PC. Concurrent FSGS and Hodgkin's lymphoma: Case report and literature review on the link between nephrotic glomerulopathies and hematological malignancies. Clin Exp Nephrol. 2006;10(4):284–289.
- Hsu SM, Waldron JW, Jr., Hsu PL, Hough AJ, Jr. Cytokines in malignant lymphomas: Review and prospective evaluation. Hum Pathol. 1993;24(10):1040–1057.
- Warnke RA, Weiss LM, Chan JKC, Cleary ML, Dorfman RF. Tumors of the Lymph Nodes and Spleen. Atlas of Tumor Pathology, Fascicle 14. 3rd series. Washington, DC: Armed Forces Institute of Pathology; 1995:53–62.
