Abstract
Gentamicin (GM), an aminoglycoside, is widely employed in clinical practice for the treatment of serious gram-negative infections. The clinical utility of GM is limited by the frequent incidence of acute renal failure. This study was designed to investigate treatment and posttreatment renoprotective potential of vitamin E and N-acetyl cysteine (NAC) against GM-induced oxidative stress and renal dysfunction. Male Sprague–Dawley rats were divided into six groups: first group is the control group that received olive oil (0.1 mL/100 g B.W.), second is the one that was treated with GM (80 mg/kg/i.p./8 days), third is the one that was treated with GM (80 mg/kg/i.p./8 days) and vitamin E (50 mg/kg/i.p./8 days), fourth is the one that was treated with GM (80 mg/kg/i.p./8 days) and NAC (50 mg/kg/i.p./8 days), fifth is the one that was treated with GM (80 mg/kg/i.p./8 days), vitamin E (50 mg/kg/i.p./8 days), and NAC (50 mg/kg/i.p./8 days), and sixth is the one that was treated with GM initially for 8 days (at 80 mg/kg/i.p.) after which vitamin E (at 50 mg/kg/i.p.) and NAC (at 50 mg/kg/i.p.) were administered for 8 days. Serum creatinine, blood urea nitrogen, serum glucose, renal malondialdehyde, renal reduced glutathione, urine sodium, fractional excretion of sodium, and histopathological examination of kidney were performed after treatment. Gentamicin treatment caused nephrotoxicity as evidenced by marked elevation in serum creatinine, blood urea nitrogen, renal malondialdehyde, urine sodium, and fractional excretion of sodium. Study of renal morphology showed marked loss of epithelium in proximal convoluted tubule, inflammatory infiltrate in the form of lymphocytes, mainly in interstitium. Treatment and posttreatment with vitamin E and NAC significantly restored renal functions, reduced lipid peroxidation, enhanced reduced glutathione level, and restored the biochemical parameters. The results of this study demonstrate the therapeutic potential of vitamin E and NAC in gentamicin-induced nephrotoxicity.
INTRODUCTION
The kidney is a particularly good target for toxins. Not only does it have a rich blood supply, receiving 25% of cardiac output, but it also helps in the excretion of these toxins by glomerular filtration and tubular secretion. Acute renal failure (ARF) occurs frequently in hospitalized patients, and it is associated with significant morbidity and mortality. The most common and generalized forms of ARF are prerenal condition and intrarenal acute tubular necrosis (ATN). Prerenal ARF in its pure state should be entirely reversible by restoring renal perfusion, but in some cases ATN has already occurred. ATN remains a more vexing problem and is seen most often with hypotension, perioperative or systemic inflammatory stresses, radiocontrast administration, and exposure to nephrotoxins.Citation1
Prerenal ARF accounts for approximately 70% of community-acquired ARF and 40% of hospital-acquired cases.Citation2 This particular form of ARF is most often due to excessive fluid deficits (hemorrhage, gastrointestinal, skin, or renal losses); however, decreased renal perfusion secondary to such pathologies as congestive heart failure, cirrhosis, or sepsis can also lead to prerenal ARF. It may progress to ATN, thus blurring the distinction between these conditions. Therapy for prerenal conditions requires prompt recognition and termination of the aggravating factor before irreversible renal cell necrosis is established.Citation3
Gentamicin, an aminoglycoside antibiotic, is widely used in the treatment of suspected or proven bacterial sepsis in newborn infants. It is rapidly bactericidal. Combined with beta-lactam antibiotics, it provides synergistic activity against the most commonly encountered pathogens in the neonatal period.Citation4
Aminoglycosides are well known for their nephrotoxic potential. Dose adjusting in renal impairment is well accepted. Regardless of proper dose adjustment, there is no guarantee of safety against nephrotoxicity, and nephrotoxicity of aminoglycosides at therapeutic levels is well established.Citation5 In clinical setup the nephrotoxicity is diagnosed very late and then the treatment is started. However, in most of the drug evaluation studies using animal models, the treatment with nephroprotective agent is initiated along with nephrotoxic agent. To match the clinical setup we therefore propose to study the effect of nephroprotective agents only after severe nephrotoxic state is attained. Current treatment of nephrotoxicity or ATN includes diuretics, growth factors, nutritional support, hydration, dopamine, fenoldopam, mannitol, calcium channel blockers, and antioxidants.
Vitamin E and N-acetyl cysteine (NAC) are well-known antioxidants. Acetylcysteine, a thiol-containing antioxidant commonly used as a mucolytic as well as an antidote for acetaminophen toxicity, shows promise as a renoprotective agent. NAC pretreatment is reported to allow CT scans in patients with ARF, which is otherwise contraindicated.Citation6 Similarly vitamin E protects unsaturated fats in the body from oxidation by peroxides and other free radicals. The possibility that vitamin E may help to prolong an active life span by slowing the rate of oxidative destruction of biological membranes and maintain the cell structural integrity is increasingly real.Citation7
Both vitamin E and NAC have been shown to provide protective effects in nephrotoxicity induced by adriamycin.Citation8 We intend to study the efficacy of both these agents in rats where significant nephrotoxicity is already observed. Thus, this investigation was undertaken to study the cotreatment and posttreatment effect of vitamin E and NAC in gentamicin-induced nephrotoxicity in rats.
MATERIALS AND METHODS
Male Sprague–Dawley rats were used for the experiment. The animals were housed under conditions of controlled temperature and 12 h day–night cycle. Animals had conventional laboratory diet and tap water ad libitum. This protocol no. KBIPER/0884 was approved during the 14th meeting of IAEC-CPCSEA held on 5 January 2008 at KBIPER, Gandhinagar. Institutional animal ethical committee (IAEC) is formed as per the Committee for the Purpose of Control and Supervision of Experiments on Animal (CPCSEA) Guidelines, Ministry of Social Justice and Empowerment, Government of India.
Drug Treatment
Gentamicin
Gentamicin sulfate injection IP (RANBIOTIC/KD-283) was obtained from Ranbaxy Laboratories, New Delhi, India.
N-acetyl cysteine
NAC (NAC/V208804, Loba Chemicals, Mumbai, India) was used for the treatment purpose.
Vitamin E
Marketed product of vitamin E (Evion 400 capsules/G0384608/MERCK, Mumbai, India) was used for treatment purpose and oil from the capsule assayed as per the procedure in Indian Pharmacopoeia 1996, Volume-II.
Assay of vitamin E
Marketed product of vitamin E (Evion capsule) was assayed as per the procedure in Indian Pharmacopoeia 1996, Volume-II. The assay value was 70.99%W/W.
Test drugs were freshly prepared.
Data analysis
Values are expressed as mean ± SEM for six animals. The results were computed statistically using the SPSS software package for windows. One-way analysis of variance followed by Tukey's test for intergroup comparisons and paired t-test was performed. p < 0.05 was considered significant.
Preparation of the test drugs
Vitamin E
0.25 mL oil + 4.75 mL olive oil
Concentration of solution: 51 mg/mL.
Dose of vitamin E: 50 mg/kg.
NAC
200 mg NAC was dissolved in 4 mL freshly boiled and cooled water.
Concentration of solution: 50 mg/mL.
Dose of NAC: 50 mg/kg.
EXPERIMENT PROTOCOL
The animals were divided into the following groups of six animals each. They were subjected to the study as described in .
Table 1. Treatment schedule
DISCUSSION
Proximal tubular epithelia are polarized cells, featuring a basolateral membrane and an apical membrane domain. Certain xenobiotics are selectively taken up by these cells via specific carriers and exported by other transmembrane transporters.Citation9 Accumulation of xenobiotics resulting in ARF is a common problem. Vitamin E as well as NAC has been shown to significantly reduce the oxidative stress in adriamycin-induced nephrotoxicity.Citation8 All aminoglycosides including gentamicin use can be complicated by well-described nephrotoxic and ototoxic effect.Citation12–14 In all these studies the protective agents were coadministered with the nephrotoxic agents. The clinical situation is quite different in majority of the cases wherein the drug treatment starts only after significant nephrotoxicity has occurred.
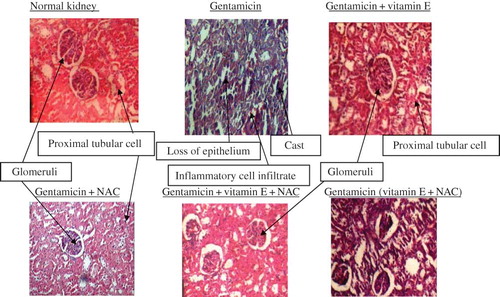
In this study we therefore aimed to compare the effect of both pre-nephrotoxicity and post-nephrotoxicity treatment of vitamin E and NAC combination. The nephrotoxicity was induced by gentamicin, a well-known antibiotic used frequently in various gram-negative infections.Citation15 In this study administration of gentamicin (80 mg/kg) for 8 days resulted in significant reduction of kidney function and also produced significant necrosis of tubular epithelia in rats. These results are in confirmation with earlier studies involving the ameliorated and healing effect of resveratrolCitation16 and protective effect of nefrolivCitation17 in gentamicin-induced nephrotoxicity at this same dose and duration of gentamicin treatment. Kidney function status in this study was evaluated by measuring various parameters such as serum creatinine, blood urea nitrogen (BUN), urine sodium levels, and fractional excretion of sodium ().
Table 2. Evaluation of kidney function by different parameters
Serum creatinine levels are significantly raised in late stage of renal damage and are therefore an important diagnostic tool even for clinically assessing kidney function (). Almost threefold higher levels of serum creatinine were observed in only-gentamicin-treated animals as compared to untreated animals. This is suggestive of significant renal damage caused by gentamicin. Even BUN levels were higher in these animals (). The renal damage produced by gentamicin involves oxidative stress. This stress is clearly reflected by higher malondialdehyde (MDA) and lower reduced glutathione (GSH) levels in these animals. MDA is a well-known marker of lipid peroxidation. Reduced GSH acts as an endogenous scavenger of peroxides converting itself to oxidized GSH. Exhaustion of GSH stores finally leads to increased cellular damage by free radicals and peroxides.Citation18,Citation19
Table 3. Result
Figure 1. Effect of vitamin E and NAC on serum creatinine levels. Notes: Each bar represents the mean ± SEM of six observations. *Significantly different from control at p < 0.05, **significantly different from model control at p < 0.05, ***significantly different from vitamin E at p < 0.05, ****significantly different from NAC at p < 0.05, *****significantly different from vitamin E + NAC at p < 0.05.
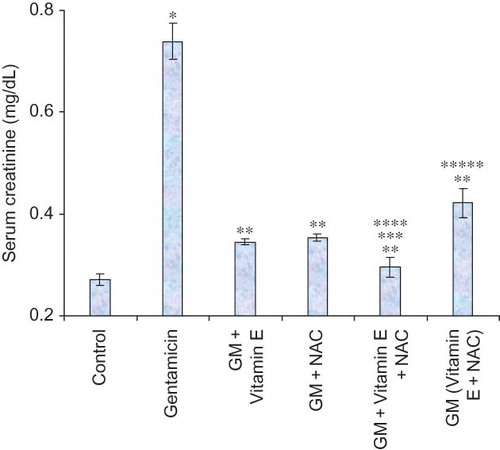
Figure 2. Effect of vitamin E and NAC on blood urea nitrogen levels. Notes: Each bar represents the mean ± SEM of six observations. *Significantly different from control at p < 0.05, **significantly different from model control at p < 0.05, ***significantly different from vitamin E at p < 0.05, ****significantly different from NAC at p < 0.05, *****significantly different from vitamin E + NAC at p < 0.05.
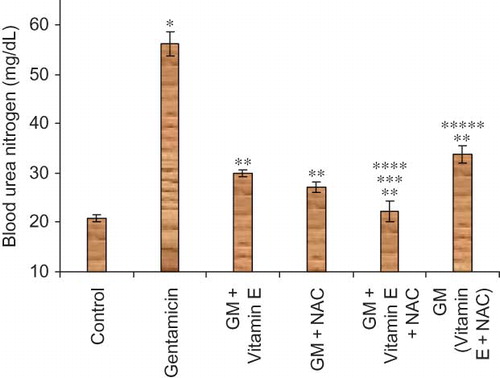
Figure 3. Effect of vitamin E and NAC on serum glucose levels. Notes: Each bar represents the mean ± SEM of six observations. *Significantly different from control at p < 0.05, **significantly different from model control at p < 0.05, ***significantly different from vitamin E at p < 0.05, ****significantly different from NAC at p < 0.05, *****significantly different from vitamin E + NAC at p < 0.05.
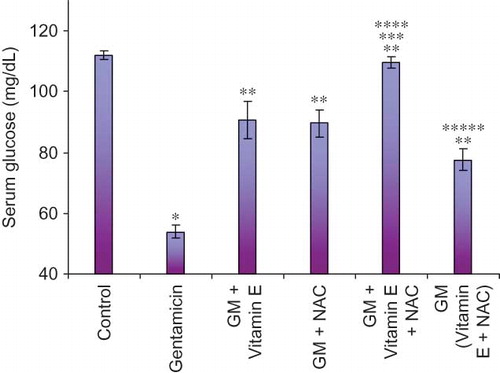
Figure 4. Effect of vitamin E and NAC on renal malondialdehyde (MDA) levels. Notes: Each bar represents the mean ± SEM of six observations. *Significantly different from control at p < 0.05, **significantly different from model control at p < 0.05, ***significantly different from vitamin E at p < 0.05, ****significantly different from NAC at p < 0.05, *****significantly different from vitamin E + NAC at p < 0.05.
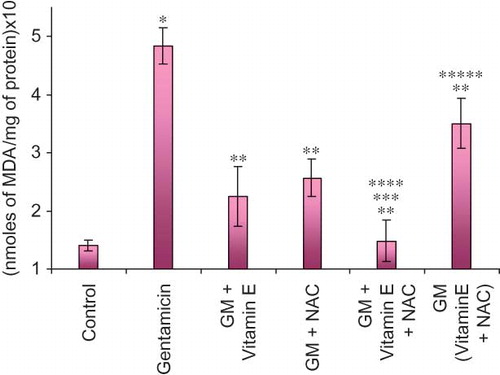
Figure 5. Effect of vitamin E and NAC on renal reduced glutathione (GSH) levels. Notes: Each bar represents the mean ± SEM of six observations. *Significantly different from control at p < 0.05, **significantly different from model control at p < 0.05, ***significantly different from vitamin E at p < 0.05, ****significantly different from NAC at p < 0.05, *****significantly different from vitamin E + NAC at p < 0.05.
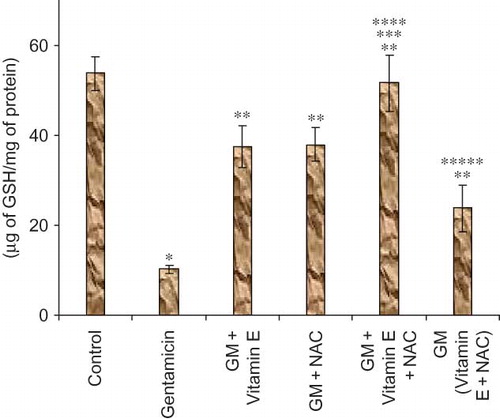
Figure 6. Effect of vitamin E and NAC on urine sodium levels. Notes: Each bar represents the mean ± SEM of six observations. *Significantly different from control at p < 0.05, **significantly different from model control at p < 0.05, ***significantly different from vitamin E at p < 0.05, ****significantly different from NAC at p < 0.05, *****significantly different from vitamin E + NAC at p < 0.05.
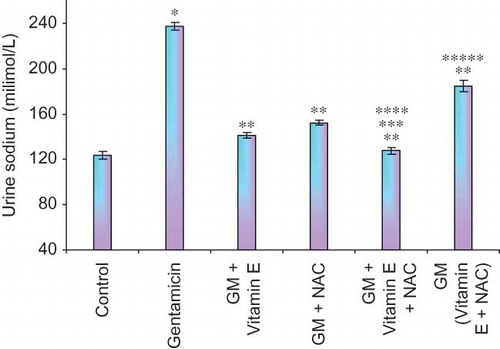
Figure 7. Effect of vitamin E and NAC on fractional excretion of sodium. Notes: Each bar represents the mean ± SEM of six observations. *Significantly different from control at p < 0.05, **significantly different from model control at p < 0.05, ***significantly different from vitamin E at p < 0.05, ****significantly different from NAC at p < 0.05, *****significantly different from vitamin E + NAC at p < 0.05.
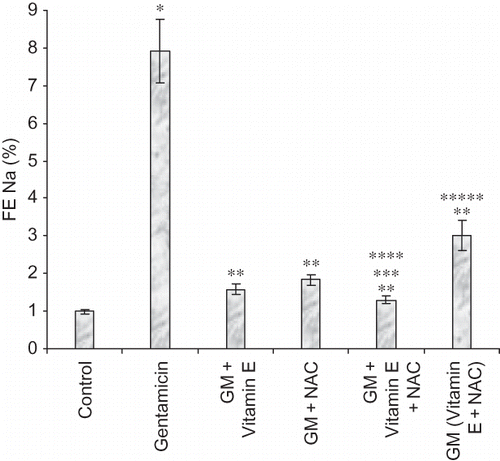
The epithelial cell damage should result in reduced reabsorption of various ions including sodium ions. In our study gentamicin-treated animals showed twice higher urine sodium levels compared to untreated animals, again suggesting significant renal damage in these animals. Compared to urine sodium levels, more accurate measure of renal damage could be a measurement of fractional excretion of sodium. Fractional excretion of sodium takes into account the physiological variations in sodium excretion and thus reduces the chances of false-positive diagnosis of renal failure. It is a direct measure of the reabsorption of filtered sodium.Citation20 Significantly high fractional excretion of sodium was observed in gentamicin-treated animals as compared to normal animals.
Hypoglycemia is one of the common complications of ARF. Aminoglycoside antibiotics impair the function of mitochondrial enzymes for the gluconeogenesis, ammoniagenesis, and tricarboxylic acid oxidation pathwaysCitation21,Citation22; in addition aminoglycoside antibiotics also downregulate the sodium-dependent glucose transport systemCitation23,Citation24 and reduce mRNA expression and protein levels of serum glutamate transaminase 1. This reduced reabsorption of glucose, resulting in increased urinary glucose excretion.Citation25 In our study, significantly lower levels of serum glucose were observed in gentamicin-treated animals as compared to normal animals.
All the above results clearly indicate that gentamicin treatment has resulted in significant ARF.
Vitamin E as well as NAC when coadministered with gentamicin to the rats showed significant protection against renal damage. Both serum creatinine and BUN levels were significantly lower in these animals as compared to gentamicin-treated animals.
We were interested in studying the post-nephrotoxicity treatment because clinically this is a more relevant situation. In our study, initiation of the treatment after completion of gentamicin treatment also showed significant improvement in renal functions as reflected by lower serum creatinine and BUN levels compared to only-gentamicin-treated animals; these effects were less pronounced than preventive therapy. As both vitamin E and NAC are well-known antioxidants, they are expected to reduce the oxidative stress. In many of the inflammatory disorders, including renal damage these drugs have shown beneficial results. In our study also, all the test drug-treated animals including the post-nephrotoxicity treated animals showed significantly lower MDA levels compared to only-gentamicin-treated animals.
In similar lines, reduced GSH levels were significantly higher in animals treated with vitamin E or NAC or both. Vitamin E and NAC in combination administered after the completion of gentamicin treatment produced similar results. In this group of animals GSH levels were significantly higher than only-gentamicin-treated animals.
Even urine sodium levels and fractional excretion of sodium were significantly lower in all the drug-treated group of animals, suggesting prevention of renal damage and/or restoration of renal function to normal level.
As mentioned earlier, hypoglycemia is one of the complications of ARF. In our study hypoglycemia produced due to gentamicin was not observed in animals coadministered with either vitamin E or NAC or both. In animals treated with vitamin E and NAC combination after ensuing of nephrotoxicity, only mild hypoglycemia was observed indicating reversal of nephrotoxicity produced by gentamicin.
Also, histological study of the kidneys showed that rats that received gentamicin had significant damage to tubular cells leading to tubular necrosis, indicating significant nephrotoxicity (). The damage to the kidneys was significantly lesser in rats that received treatment with vitamin E or NAC or their combination as cotreatment or posttreatment. These results are in confirmation with the reports showing efficacy of vitamin E and NAC combination in adriamycin-induced nephrotoxicity.Citation8
Thus our result from this study is clearly indicative of nephroprotective effects of vitamin E and NAC either alone or in combination. The combination treatment seems to be more effective than single-agent treatment. The combination when administered after the maximal renal damage was produced by gentamicin showed significant restoration of renal function.
Many of the commonly prescribed medications such as amphotericin B,Citation26 cyclosporine,Citation27 angiotensin converting enzyme inhibitors,Citation28 or vancomycinCitation29 are known nephrotoxic agents. They share the same mechanism which involves generation of reactive oxygen species either through renal hypoperfusion or ischemia in producing ATN. Both vitamin E and NAC by virtue of their antioxidant property provide protection against reactive oxygen species induced nephrotoxicity. Our study showing efficacy of vitamin E and NAC against gentamicin-induced nephrotoxicity can therefore be extrapolated to nephrotoxicity caused by all the above-described agents.
CONCLUSION
Vitamin E and NAC alone or in combination provide significant protection against nephrotoxicity. The combination is even effective when administered after maximal damage with gentamicin was produced. This protective effect is most likely to be due to their antioxidant property.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Dishart MK, Kellum JA. An evaluation of pharmacological strategies for the treatment and prevention of acute renal failure. Adis Int Drugs. 2000;59(1):79–91.
- Albright RC. Acute renal failure a practical update. Mayo Clin Proc. 2001;76:6–74.
- Smith S, Marriott J. Acute renal failure. In: Walker R, Edwards C, eds. Acute Renal Failure: Pathophysiology, Diagnosis, and Management. 3rd ed., Edinburgh: Churchill Livingstone; 2003:229–245.
- Chattopadhyay B. Newborns and gentamicin- how much and how often? J Antimicrob Chemother. 2002;49:13–16.
- Smith CR, Lipsky JJ, Laskin OL, Double-blind comparison of the nephrotoxicity and auditory toxicity of gentamicin and tobramycin. N Engl J Med. 1980;302(20):1106–1109.
- Tulkens PM. Experimental studies on nephrotoxicity of aminoglycosides at low doses; mechanisms and perspectives. Am J Med. 1986;80:105–114.
- Dominguez JH, Hale CC, Qulali M. Studies of renal injury. Gentamicin toxicity and expression of basolateral transporters. Am J Physiol. 1996;270:F245–F253.
- Horio M, Fukuhara Y, Orita Y, . Gentamicin inhibits Na+ dependent D-glucose transport in rabbit kidney brush- border membrane vesicles. Biochem Biophys Acta. 1986;858:153–160.
- Takamoto K, Kawada M, Usui T, Ishizuka M, Ikeda D. Inhibitory activity of dome formation in LLC PK1 cells is a selective index of aminoglycoside nephrotoxicity. J Antibiot (Tokyo). 2002;55(6):605–612.
- Branch RA. Prevention of amphotericin B-induced renal impairment: A review on the use of sodium supplementation. Arch Intern Med. 1988;148:2389–2396.
- Wang C, Salahudeen AK. Lipid peroxidation accompanies cyclosporine nephrotoxicity: Effects of vitamin E. Kidney Int. 1995;47:927–934.
- Albright RC. Acute renal failure a practical update. Mayo Clin Proc. 2001;76:6–74.
- Evenepoel P. Acute toxic renal failure. Best Pract Res Clin Anesthesiol. 2004;18(1):37–52.
- Humes DH, Weinberg JM, Knauss TC. Clinical and pathophysiologic aspects of aminoglycoside nephrotoxicity. Am J Kidney Dis. 1982;2:5–29.
- Tulkens PM. Nephrotoxicity of aminoglycoside antibiotics. Toxicol Lett. 1989;46:107–23.
- Coskun S, Ozge U, Nil UC, Sanem G, Selma B, Mujgan C. Gentamicin-induced nephrotoxicity in rats ameliorated and healing effects of resveratrol. Biol Pharma Bull. 2007;30:79–83.
- Meher SK, Chaudhuri SB, Marjit B, Munshi S. Nephroprotective effect of nefroliv in gentamicin-induced nephrotoxicity. Phytomedica. 2001;2(1&2):41–8.
- Baliga R, Ueda N, Walker, PD, Shah SV. Oxidant mechanisms in toxic acute renal failure. Am J Kidney Dis. 1997;29:465–77.
- Meister A. Glutathione metabolism and its selective modification. J Biol Chem. 1988; 263(33):17205–8.
- Steiner R. Interpreting the fractional excretion of sodium. Am J Med. 1984;77(4):699–702.
- Sandhya P, Mohandass S, Varalakshmi P. Role of dl-alpha lipoic acid in gentamicin induced nephrotoxicity. Mol Cell Biochem. 1995;145:11–17.
- Tulkens PM. Experimental studies on nephrotoxicity of aminoglycosides at low doses; mechanisms and perspectives. Am J Med. 1986;80:105–14.
- Dominguez JH, Hale CC, Qulali M. Studies of renal injury. Gentamicin toxicity and expression of Basolateral transporters. Am J Physiol. 1996;270:F245–53.
- Horio M, Fukuhara Y, Orita T, . Gentamicin inhibits Na+ dependent D-glucose transport in rabbit kidney brush- border membrane vesicles. Bio Chem Biophys Acta. 1986;858:153–60.
- Takamoto K, Kawada M, Usui T, Ishizuka M, Ikeda D. Inhibitory activity of dome formation in LLC PK1 cells is a selective index of aminoglycoside nephrotoxicity. J Antibiot (Tokyo) 2002;55(6):605–12.
- Branch RA. Prevention of amphotericin B-induced renal impairment: a review on the use of sodium supplementation. Arch Intern Med. 1988;148:2389–96.
- Wang C, Salahudeen AK. Lipid peroxidation accompanies cyclosporine nephrotoxicity: Effects of vitamin E. Kidney Int 1995;47:927–34.
- Albright RC. Acute renal failure a practical update. Mayo Clin Proc 2001;76:6–74.
- Evenepoel P. Acute Toxic Renal Failure. Best Pract Res Clin Anesthesiol 2004;18(1):37–52.