Abstract
The aim of this study is to test the glomerular and other quantitative parameters of kidneys of anencephalic fetuses and comparing those to “normal” fetuses. In this study, 20 kidneys of human fetuses (5 boys and 5 girls of anencephalic fetus, and 5 boys and 5 girls of normal fetus), at gestational ages of 25–30 weeks, were examined. This study is based on two basic research methods: one is a conventional anatomical measurement at the macroscopical level; the other is a design-biased stereological method at the microscopical level. Physical dissector and Cavalieri principle were used to estimate the total and numerical density of glomerulus and the volume of kidney, respectively. The results of the two types of investigation were compared based on anencephalic/normal and boy/girl kidneys at both the macroscopical and microscopical levels. There was no significant difference found between the quantitative features of kidneys (volume of kidneys and mean number and/or height of glomerulus) belonging to anencephalic and normal fetuses. The results of this study suggest that anencephalic fetuses did not differ from normal fetuses in respect of kidneys.
INTRODUCTION
Quantitative features of normal and anencephalic kidneys of fetuses at the gestational ages of 25–30 weeks are not well known. Morphological features of this organ belonging to these fetuses may gain a substantial audience, since the transplantation requirements for various organs are increasing in modern life; for this reason, researchers have focused on potential alternative organ sources such as adult or infant cadavers and anencephalic fetuses. Anencephaly, a neural tube defect, is one of the most frequently seen embryonic malformations. Although its etiology has not been clearly understood, some findings from experiments carried out on chick and mouse embryos showed that it may root from genetic disorders.Citation1,Citation2 A group of genes have been implicated in the networking of crest cell induction, migration, specification, and differentiation.Citation3,Citation4 Many system malformations in the body of a fetus are also associated with neural tube defects. Several neural and non-neural malformations, such as cardiovascular abnormalities, anencephaly with cranioschisis, and spina bifida aperta with myeloschisis, are also commonly associated with neural tube defects.Citation5,Citation6 To our knowledge, there are no reports on kidney malformations that are rooted from anencephaly and it has been claimed that kidneys from an anencephalic fetus are an acceptable source for transplantation.Citation7–10 New organ sources for transplantation, in spite of some limitations such as the paucity of organs, tissue rejection, technical problems, and current law, compelled the researcher to investigate new sources for organ donation that can help meet the demand for transplantation.Citation11–14
Stereology is a series of techniques that are based on well-defined mathematical and statistical background. The data accumulated through these methods are based on two-dimensional images of three-dimensional structures.Citation15,Citation16 The number and size of the nephrons, which are the functional units of a kidney, and other quantitative data of this organ, such as volume, give us very important information about the function and organization of the kidney. In the present study, we attempted to determine not only the number and size of glomeruli by the dissector-counting method, but also the total volume of the kidney using the Cavalieri principle in anencephalic and normal fetuses.Citation17,Citation18
MATERIALS AND METHODS
Experimental Design
This study was approved by the Human Ethic Committee of the Medical School of Karadeniz Technical University. Twenty of 25–30-week-old fetuses were used in this study. The age of fetuses was determined according to these criteria: (1) crown-rump length was 250–280 mm; (2) biparietal diameter was 55–63 mm; and (3) weight of fetus was 1000–1700 g. Samples were collected from the gynecology clinic from 1995 to 1998. Gross developmental features (normal or anencephalic) and anatomical characteristics (kidney weight, length, etc.) were determined at the Department of Anatomy. The kidneys were divided into four groups: normal boy (n = 5), normal girl (n = 5), anencephalic boy (n = 5), and anencephalic girl (n = 5). No developmental abnormalities were observed at the macroscopic level in the normal fetuses and there were no developmental abnormalities in the anencephalic fetuses except for the anencephaly. It was decided whether a fetus was anencephalic or not based on the absence of brain and calvarium superior to the orbits. The macroscopical measurements were done before tissue fixation as shown in .
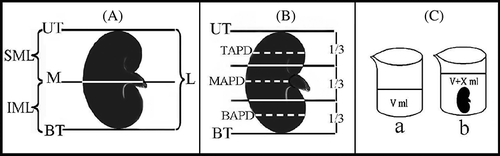
Figure 1. (A)–(C) show macroscopic measurements that were made on the kidneys.Note: UT, upper top; BT, bottom top; M, median line; SML, superior–midline length; IML, inferior–midline length; L, length; TAPD, top anterior–posterior depth; MAPD, middle anterior–posterior depth; BAPD, bottom anterior–posterior depth; a and b show fluid displacement for volume measurements of kidney.
Tissue Preparation Procedures
Twenty kidneys from fetuses (five boys and five girls anencephalic; five boys and five girls normal) weighing between 1000 and 1700 g at gestational ages between 25 and 30 weeks were studied in this experiment. All chemicals used during the study were obtained from Merck Chemical Company (Merck Chemicals, Darmstadt, Germany). Kidneys were fixed by 10% formaldehyde; after fixation they were dehydrated through graded alcohol series and embedded in paraffin wax. Serial sections of 5 µm thickness at transverse plane were taken and mounted onto gelatinized glass slides and then stained with hematoxylin–eosin.
Tissue Sampling and Stereological Methods
On the basis of a pilot study, we obtained approximately 1800 sections from a kidney. We decided to select every 31st specimen and its adjacent sections as dissector pairs from the tissue sections using a systematic random sampling approach. As a result of this sampling strategy, approximately 55–60 section pairs for each kidney were used. The physical dissector-counting method was applied to these section pairs to estimate the total number of glomeruli in each kidney. One of the section pairs was used for kidney volume estimation. The first selected section and its adjacent section, called the dissector pair, were separated by a 30 µm distance (). A stereology analysis system (Stereoinvestigator 6.0, Microbrightfield, Colchester, VT, USA) was used for the stereological estimation of the kidneys' volume and number of glomeruli. The level of coefficient of errors (CEs) for number of glomeruli and kidney volume estimation was determined to be in an acceptable range.Citation19
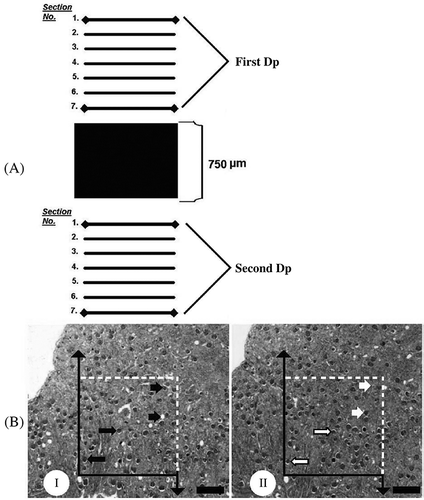
Figure 2. (A) Sampling strategy that is used at the section level; (B) (I) reference and (II) look-up sections of the dissector pair are seen. The distance between them is 30 µm. These pairs are used for the estimation of both the number and height of glomeruli. Long arrows show glomeruli that are seen at both the reference and look-up sections; short black arrows point to dissector glomeruli that are observed in the reference section but not in the look-up section (white short arrow in B). Dp is a dissector pair. Scale bar is 500 µm.
Volume Estimation with the Cavalieri Principle
Volume estimation of a structure that has an arbitrary shape and size may be efficiently obtained by the Cavalieri principle.Citation20 An important rule of this principle is as follows: in order to get an unbiased estimation of the volume of an object, the object must be cut to serial and parallel planes separated by a fixed distanceCitation21,Citation22 and using a point-counting grid for the area estimation of section profiles. The point density of the grid was designed to obtain an appropriate CE for the serial paraffin sections of the study. The CE and coefficient of variation were estimated according to the formula given by Baddeley and Jensen in 2005.Citation19
The test grid with a systematic array of points is randomly placed on the screen of a PC and an appropriate point per interested area (total kidney areas) carefully calculated for each section. The volumes of the kidneys were estimated with the following formula:
where is the mean volume of the kidney, t the mean section thickness, a/p the inter-point area, and ∑P the total number of points hitting whole serial sections of the kidney.
Estimation of Glomerulus Number
The boundary of a section that belongs to the dissector pair called the reference section was traced with Stereoinvestigator software and then it was used to determine the section's cut surface area. The estimated reference section area of each kidney profile was divided into equal fields in the x and y-axes of the microscope. Finally, the images of all fields in each step, as determined previously via motorized stages of the microscope, were taken using a charged-coupled device (CCD) camera. The same procedure was applied to the other section of the section pair, called the look-up section ( and ). At this point, a major difficulty for the researcher is to provide an identical orientation for the same areas in the dissector pair. After the same fields were obtained, images of the dissector pairs were transferred to another PC. First, the adjacent fields were located on the PC screen. Second, a suitable unbiased counting frame was manually placed on the same structures of these sections with a fixed rule. Finally, the dissector-counting method was applied to these section pairs. Reference and look-up sections were reversed in order to double the number of dissector pairs without taking new sections ( and ).
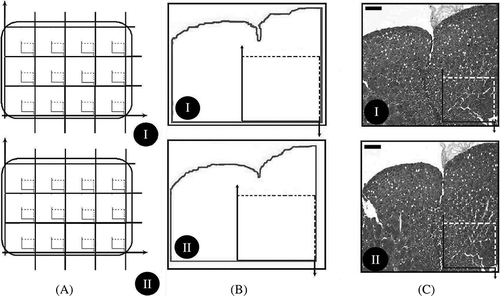
Figure 3. (A) (I, II) Illustration of section area fraction strategy. On average, 12–14 section pairs were obtained at section level and divided into approximately 90 equal areas. (B) (I, II) An illustration of area fraction strategy. Unbiased counting frames were put on images. (C) (I, II) Micrographs of a dissector pair are seen that are used for estimating the number and height of a glomerulus.
The mean numerical density of glomeruli (NVglo) was estimated using the following formulaCitation23,Citation24:
where is the total number of counted glomeruli seen in the reference sections but not in the look-up sections, t the mean section thickness, and A the area of the counting frame.
The mean glomerulus height (Hglo) was estimated with the following equation24:
where is the total number of glomeruli seen in the reference section and
the total number of dissector glomeruli that appeared only in the reference section.
The CEs for the volume and glomeruli height and number estimation and the coefficient of variation for all data were calculated according to Gundersen and Jensen's formula.Citation20,Citation22
Statistical Analysis
Differences in the number/height of glomeruli and the volume of kidneys between the two groups were tested using the Mann–Whitney U-test. All statistical analyses were performed using SPSS 13.0 software for Windows.
RESULTS
Macroscopical Results
The macroscopical results for the anatomical properties of the kidneys are given in . No significant differences were found between the normal and anencephalic boys and between normal and anencephalic girls; normal or anencephalic left and right kidneys; or regarding any other anatomical features of kidneys (p > 0.05)
Table 1. Data belong to the dimensions, weight, and volumes of right and left kidneys (mean ± SEM)
Stereological Results
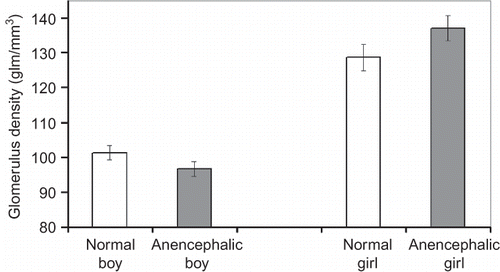
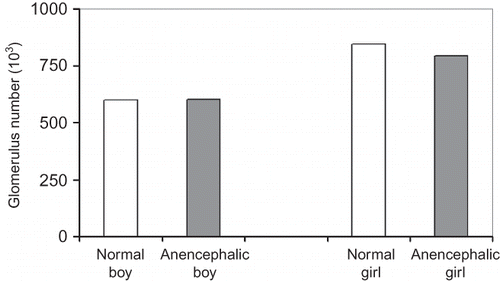
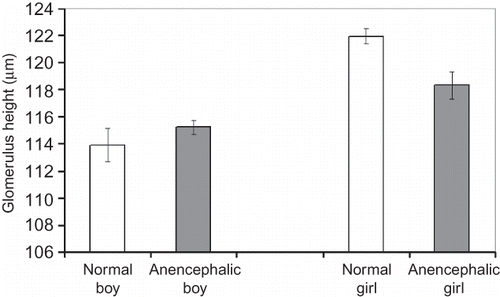
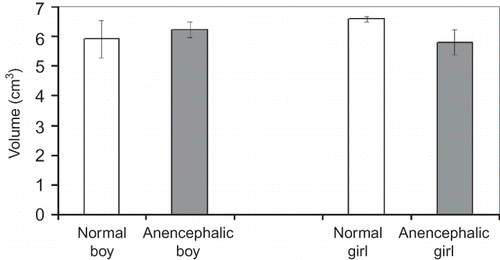
The density of glomeruli, the number and height of glomeruli, and the volume of the kidneys were compared as shown below:
Male–female (irrespective of normal or anencephalic)
Normal–anencephalic (irrespective of male or female)
Normal fetuses and anencephalic fetuses (regardless of gender)
Male anencephalic fetuses–male normal fetuses
Female anencephalic fetuses–female normal fetuses
No significant differences in glomerulus density, total glomerulus number, mean glomerulus height, and mean kidney volume were detected between the normal fetuses and anencephalic fetuses regardless of gender (p > 0.05) (Figures ). Also there was no statistical difference, either between normal and anencephalic male fetuses or between normal and anencephalic female fetuses in respect of morphometrical kidney parameters (p > 0.05).
Histological Results
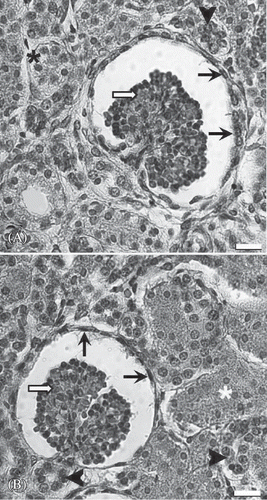
The renal cortex of the anencephalic and normal fetuses contained glomeruli, vessels, tubules, and interstitium ( 8A and B). When evaluating these renal specimens by light microscopy on a hematoxylin–eosin-stained section, the following glomerular features were seen: the overall cellularity of the glomerulus, the symmetry of the glomerulus, and the thickness of the capillary walls (). Renal tubule (the long and winding neck) formed the proximal tubule, the loop of Henle, and the distal tubule (). Among tubular structures, there was very little interstitium in the cortex (). So, the general histological structure of anencephalic kidney was the same compared with the kidney of normal fetuses at light microscopic level.
Figure 8. Representative light micrographs of renal cortex from a normal fetus (A) and an anencephalic fetus (B). The structure of the anencephalic kidney does not show a significant difference from the normal kidney. Arrow head, distal tubule; asterisk, proximal tubule; white arrow, glomerulus; black arrow, visceral sheet of Bowman capsule. Scale bar is 40 µm.
DISCUSSION
Clinically, it is an important issue to acquire appropriate donors for kidney transplantation in pediatric patients who suffer from chronic kidney disease, which has a significant morbidity and mortality rate. Currently, it has been argued whether anencephalic fetuses (occurring in 1:1000–1:20,000 infants) would make ideal donors. In the literature, there are many studies about this subject. Some of these studies declared a positive opinion and successful results.Citation25–27 On the other hand, some of them mentioned some disadvantages, such as effect of anencephaly on other organs,Citation28 arterial thrombosis, vascular rejection,Citation8 the size of the recipient (age and sex),Citation29 and ethical problems,Citation30 due to no more than a few weeks of life expectancy for anencephalic infants.Citation14 In this study, it was attempted to detect the glomerular and other quantitative parameters of kidneys of anencephalic and normal fetuses.
The urinary system of a developing fetus appears at three stages: pronephros, mesonephros, and metanephros. The development of both the number and size of nephrons continues throughout the fetal stage and the generation of the nephrons is completed between the 28th and 36th week of gestation, but the structural and functional maturation of the nephron continues after birth.Citation31 In order to determine whether kidney of anencephalic and normal fetuses is functional, the number, shape, size, and distribution of nephronsCitation32,Citation33 and the total volume of the kidneyCitation34 and its anatomical characteristics should be evaluated.Citation35,Citation36
Although there are many studies investigating the features of the kidneyCitation37,Citation38 using stereological methods, there are few studies on kidney developmentCitation37,Citation39–44 and none of these specifically study anencephalic fetus kidneys.
It is well known that even though a few mature nephrons are observed from the ninth week of gestation, most of them are formed during the third trimester.Citation45,Citation46 The stereological findings of the present study show that the number of nephrons at 25–30 weeks of gestation were not different between normal male and female fetuses (p > 0.05). In addition, no differences were found between the number of glomeruli of normal and anencephalic fetuses. Therefore, the number of glomeruli in the kidney is not affected by anencephaly of the fetus. The second finding was obtained with the physical dissector method. There were no significant differences between the mean glomerular heights of normal and anencephalic male or female fetuses. The third finding of this study involves the total volume of the kidneys. These results also show no significant differences between the mean volumes of normal and anencephalic male or female fetuses. Our data regarding the mean kidney volume at the same period were parallel with previous findings that there is no difference between male and female kidneys.Citation47,Citation48 Our studies on the glomerular surface area, glomerular volume, total length of glomerular capillary, and so on, of the normal and anencephalic fetuses using stereological methods are continuing. The present findings of this study show that the kidneys of anencephalic fetuses have similar histological structure and morphological parameters with those of normal fetuses.
ACKNOWLEDGMENTS
This research was conducted in the Laboratory of Department of Histology and Embryology, School of Medicine, Ataturk University, Erzurum, Turkey, and the Laboratory of Anatomy, School of Medicine, Karadeniz Technical University, Trabzon, Turkey. None of the authors has commercial interests, financial interests, and/or other relationships with manufacturers of pharmaceuticals, laboratory supplies, and/or medical devices or with commercial providers of medically related services.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Copp AJ, Greene ND, Murdoch JN. The genetic basis of mammalian neurulation. Nat Rev Genet. 2003;4:784–793.
- Juriloff DM, Harris MJ, Tom C, MacDonald KB. Normal mouse strains differ in the site of initiation of closure of the cranial neural tube. Teratology. 1991;44:225–233.
- O'Rahilly R, Muller F. Interpretation of some median anomalies as illustrated by cyclopia and symmelia. Teratology. 1989;40:409–421.
- O'Rahilly R, Muller F. The two sites of fusion of the neural folds and the two neuropores in the human embryo. Teratology. 2002;65:162–170.
- Campbell LR, Dayton DH, Sohal GS. Studies on the etiology of neural tube defects. Teratology. 1986;34:171–187.
- Padmanabhan R. Etiology, pathogenesis and prevention of neural tube defects. Congenit Anom Kyoto. 2006;46:55–67.
- Borboroglu PG, Foster CE, Philosophe B, . Solitary renal allografts from pediatric cadaver donors less than 2 years of age transplanted into adult recipients. Transplantation. 2004;77:698–702.
- Gomez-Campdera FJ, Robles NR, Anaya F, . Kidney transplantation from anencephalic donors. Report of 5 cases and a review of the literature. Child Nephrol Urol. 1990;10:143–149.
- Gutierrez-Carreno R, Lehne-Garcia C, Ojeda-Duran S, . Renal transplants from anencephalic donors. Bol Med Hosp Infant Mex. 1989;46:808–811.
- Iitaka K, Martin LW, Cox JA, McEnery PT, West CD. Transplantation of cadaver kidneys from anencephalic donors. J Pediatr. 1978;93:216–220.
- Gutierrez Calzada JL, Martinez JL, Baena V, . En bloc kidney and bladder transplantation from an anencephalic donor into an adult recipient. J Urol. 1987;138:125–126.
- Kinnaert P, Vereerstraeten P, Van Asperen de Boer F, . Transplantation of both kidneys of an anencephalic newborn to a 23-year-old patient. Eur Urol. 1981;7:373–376.
- Ohshima S, Ono Y, Kinukawa T, Matsuura O, Tsuzuki K, Itoh S. Kidney transplantation from an anencephalic baby: A case report. J Urol. 1984;132:546–547.
- Peabody JL, Emery JR, Ashwal S. Experience with anencephalic infants as prospective organ donors. N Engl J Med. 1989;321:344–350.
- Mayhew TM. Stereology and the placenta: Where's the point? A review. Placenta. 2006;27:17–25.
- Nyengaard JR. Stereologic methods and their application in kidney research. J Am Soc Nephrol. 1999;10:1100–1123.
- Nyengaard JR, Bendtsen TF. Glomerular number and size in relation to age, kidney weight, and body surface in normal man. Anat Rec. 1992;232:194–201.
- Sahin B, Emirzeoglu M, Uzun A, . Unbiased estimation of the liver volume by the Cavalieri principle using magnetic resonance images. Eur J Radiol. 2003;47:164–170.
- Baddeley A, Jensen EBV. Stereology for Statisticians. Boca Raton, FL: Chapman & Hall/CRC; 2005.
- Gundersen HJ, Jensen EB. The efficiency of systematic sampling in stereology and its prediction. J Microsc. 1987;147:229–263.
- Cruz-Orive LM, Weibel ER. Recent stereological methods for cell biology: A brief survey. Am J Physiol. 1990;258:148–156.
- Gundersen HJ. Stereology of arbitrary particles. A review of unbiased number and size estimators and the presentation of some new ones, in memory of William R. Thompson. J Microsc. 1986;143:3–45.
- Cruz-Orive LM, Geiser M. Estimation of particle number by stereology: An update. J Aerosol Med. 2004;17(3):197–212.
- Sterio DC. The unbiased estimation of number and size of arbitrary particles using the dissector. J Microsc. 1984;134:127–136.
- Fernandez LA, Turgeon NA, Odorico JS, . Superior long-term results of simultaneous pancreas-kidney transplantation from pediatric donors. Am J Transplant. 2004;4:2093–2101.
- Ruff T, Reddy KS, Johnston TD, . Transplantation of pediatric en bloc cadaver kidneys into adult recipients: A single-center experience. Am Surg. 2002;68:857–859.
- Smyth GP, Eng MP, Power RP, Hickey DP, Little DM. Long-term outcome of cadaveric pediatric en bloc transplantation-a 15-year experience. Transplant Proc. 2005;37:4228–4229.
- Nakanishi T, Yamamoto M. Macroscopical observations on a human anencephalous fetus with multiple malformations. Hokkaido Igaku Zasshi. 1978;52:285–304.
- Feltran Lde S, Nogueira PC, Bocaletti AP, Christofalo DM, Ajzen SA, Pacheco-Silva A. Assessment of factors determining graft size in transplant of cadaver kidneys from child donors. Transplantation. 2005;79:1731–1736.
- Spital A. Ethical and policy issues in altruistic living and cadaveric organ donation. Clin Transplant. 1997;11:77–87.
- Čukuranović R, Vlajković S. Age related anatomical and functional characteristics of human kidney. Facta Univ Ser Med Biol. 2005;12:61–69.
- Dakovic-Bjelakovic M, Vlajkovic S, Cukuranovic R, Antic S, Bjelakovic G, Mitic D. Quantitative analysis of the nephron during human fetal kidney development. Vojnosanit Pregl. 2005;62:281–286.
- Gross ML, Amann K, Ritz E. Nephron number and renal risk in hypertension and diabetes. J Am Soc Nephrol. 2005;16:27–29.
- Vlajkovic S, Dakovic-Bjelakovic M, Cukuranovic R, Popovic J. Evaluation of absolute volume of human fetal kidney's cortex and medulla during gestation. Vojnosanit Pregl. 2005;62:107–111.
- Chitty LS, Altman DG. Charts of fetal size: Kidney and renal pelvis measurements. Prenat Diagn. 2003;23:891–897.
- Cohen HL, Cooper J, Eisenberg P, . Normal length of fetal kidneys: Sonographic study in 397 obstetric patients. Am J Roentgenol. 1991;157:545–548.
- Gubhaju L, Black MJ. The baboon as a good model for studies of human kidney development. Pediatr Res. 2005;58:505–509.
- Razga Z, Nyengaard JR. A stereologic approach to estimate the number of immunogold-labeled molecules in cells of tubules. Anal Quant Cytol Histol. 2006;28:54–60.
- Armitage JA, Lakasing L, Taylor PD, . Developmental programming of aortic and renal structure in offspring of rats fed fat-rich diets in pregnancy. J Physiol. 2005;565: 171–184.
- Bendtsen TF, Nyengaard JR. Unbiased estimation of particle number using sections – An historical perspective with special reference to the stereology of glomeruli. J Microsc. 1989;153:93–102.
- Naprstkova I, Radochova B, Novotna B, Jirkovska M, Janacek J, Kubinova L. Quantitative analysis of embryonic kidney impairment by confocal microscopy and stereology: Effect of 1,2-dibromoethane in the chick mesonephros. Br Poult Sci. 2005;46:661–667.
- Razga Z. Three-dimensional morphologic examination of normal and diseased renal arterioles. Orv Hetil. 2003;144:2165–2172.
- Rieder MJ, Roman RJ, Greene AS. Reversal of microvascular rarefaction and reduced renal mass hypertension. Hypertension. 1997;30:120–127.
- Wintour EM, Moritz KM, Johnson K, Ricardo S, Samuel CS, Dodic M. Reduced nephron number in adult sheep, hypertensive as a result of prenatal glucocorticoid treatment. J Physiol. 2003;549:929–935.
- Haycock GB. Development of glomerular filtration and tubular sodium reabsorption in the human fetus and newborn. Br J Urol. 1998;81:33–38.
- Hinchliffe SA, Sargent PH, Howard CV, Chan YF, van Velzen D. Human intrauterine renal growth expressed in absolute number of glomeruli assessed by the dissector method and Cavalieri principle. Lab Invest. 1991;64:777–784.
- Hsieh YY, Chang CC, Lee CC, Tsai HD. Fetal renal volume assessment by three-dimensional ultrasonography. Am J Obstet Gynecol. 2000;182:377–379.
- Sampaio FJ. Analysis of kidney volume growth during the fetal period in humans. Urol Res. 1992;20:271–274.