Abstract
Anti-glomerular basement membrane (GBM) antibody disease is clinically manifested as rapidly progressive glomerulonephritis (RPGN) with crescentic changes. The renal prognosis is poor. We report here the case of a 61-year-old woman with myeloperoxidase antineutrophil cytoplasmic antibody (MPO-ANCA)-positive anti-GBM antibody disease. This patient was referred to our hospital because of RPGN. Anti-GBM antibody was positive with a titer of 38 EU. The MPO-ANCA titer was 65 EU. Chest imaging examination revealed pulmonary multiple nodules. ANCA-associated vasculitis was suspected. Renal pathology revealed cellular crescents in 13 out of 17 glomeruli. Immunofluorescence with anti-IgG antibody, anti-C3 antibody, and anti-fibrin antibody showed linear staining along the glomerular capillary walls. Based on these findings, the patient was diagnosed with anti-GBM antibody disease. Hemodialysis was started because of uremic syndrome with elevated serum creatinine (6.84 mg/dL). In addition, treatment with plasma exchange using 3.6 L (90 mL/kg) of fresh frozen plasma combined with an oral dose of 40 mg of prednisolone was initiated. Within 3 weeks, both types of autoantibodies became undetectable. Subsequently, this patient achieved dialysis independence and remission of glomerulonephritis. No adverse effects were observed. In patients with MPO-ANCA-positive anti-GBM antibody disease, intensive therapy predominantly with plasma exchange might be operative, even though renal function is less likely to recover.
INTRODUCTION
Anti-glomerular basement membrane (GBM) antibody disease is a rare autoimmune disease characterized by anti-GBM antibody-mediated glomerulonephritis. Renal function rapidly deteriorates in this disorder. Renal biopsy shows crescentic glomerulonephritis and linear deposition of IgG along the glomerular capillary walls. Patients with alveolar hemorrhage and severe renal failure should be considered for urgent immunosuppressive therapy combined with plasmapheresis to maximize the chance of renal recovery.Citation1–3 However, patients needing immediate dialysis are less likely to recover.Citation3 Therapy may elicit potentially serious complications, such as intercurrent infection.Citation4,5 Previous reports have revealed that up to 40% of patients with anti-GBM antibody disease also presented positive for circulating antineutrophil cytoplasmic antibody (ANCA) and that myeloperoxidase (MPO)-ANCA was predominant.Citation4,6–8 Furthermore, the presence of ANCA might predict the renal prognosis.Citation4,6,9,10
In this report, we describe a case of chronic renal failure due to MPO-ANCA-positive anti-GBM antibody disease that was successfully treated by plasma exchange and immunosuppressive therapy.
CASE REPORT
A 61-year-old woman was admitted to our hospital because of loss of appetite and renal dysfunction. Ten days prior to the admission, she developed fever and microhematuria. After presenting to her community hospital, a diagnosis of pyelonephritis was suspected. Oral antibiotics were initiated, but her symptoms did not improve. Three days prior to the admission, she was found to have renal dysfunction with proteinuria, hematuria, and granular cast. Previous renal function had been normal. Thus, rapidly progressive glomerulonephritis (RPGN) was suspected and the patient was referred to our hospital.
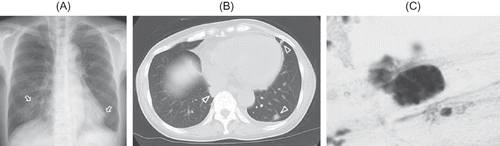
On admission, her height was 143 cm, weight was 38.8 kg, blood pressure was 99/47 mmHg, and body temperature was 37.4°C. Oxygen saturation was 98%. She had no preceding episodes of infection. She was not a smoker. Physical examination revealed anemic conjunctivae. Laboratory findings indicated anemia, leukocytosis, renal dysfunction, microhematuria, proteinuria, and an elevated serum level of C-reactive protein (). Both anti-GBM antibody and MPO-ANCA were positive. Antinuclear antibody was negative, and complement levels were normal. Electrocardiogram was normal. Chest radiograph revealed a few small obscure nodular shadows in bilateral lung fields (A, arrows). Chest computed tomography (CT) examination showed multiple nodules (B, arrow heads). She had neither a cough nor hemoptysis. To clarify the etiology of the nodules in the lung, serological tests were performed and sputum culture was collected. On the seventh day of hospitalization, bronchoscopy with broncho-alveolar lavage was performed. The cultures of sputum and broncho-alveolar lavage were negative. Cytology of the broncho-alveolar lavage showed hemosiderin phagocytosis by macrophages (C). These findings excluded pulmonary infective diseases and malignant diseases. Anti-GBM antibody disease occasionally develops alveolar hemorrhage, but typically manifests as patchy or diffuse opacities in chest imaging test. However, chest CT examination, which suggests the diagnosis of vasculitis, shows various findings including nodular shadow. Collectively, the most likely cause of the nodules in the lung was ANCA-associated vasculitis.
Table 1. Laboratory findings on admission.
Figure 1. (A) Chest radiograph showing a few small obscure nodular shadows in bilateral lung (arrows). (B) Chest computed tomography examination showing multiple nodules (arrow heads). (C) Macrophages phagocytizing hemosiderin by cytology of broncho-alveolar lavage.
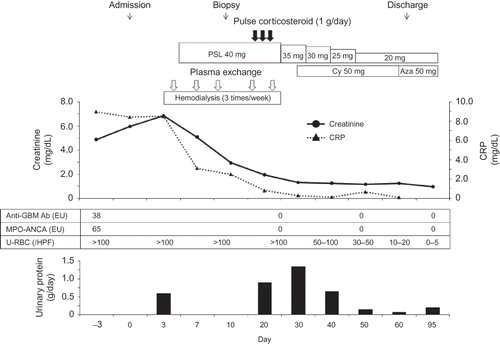
The patient’s clinical course is shown in . Within 72 h of admission, hemodialysis was started, because of uremic syndrome. On the fifth day of hospitalization, an oral dose of 40 mg of prednisolone (1 mg/kg) and plasma exchange were initiated since she was suspected of having pulmonary-renal syndrome. At this point, because pulmonary infective diseases had not been ruled out, neither pulse methylprednisolone nor cytotoxic drugs were administered. For every plasma exchange, 3.6 L (90 mL/kg) of fresh frozen plasma was given as the replacement fluid.
Figure 2. Clinical course.
Note: MPO-ANCA, myeloperoxidase antineutrophil cytoplasmic antibody; GBM, glomerular basement membrane; PSL, predonisolone; Cy, cyclophosphamide; Aza, azathioprine.
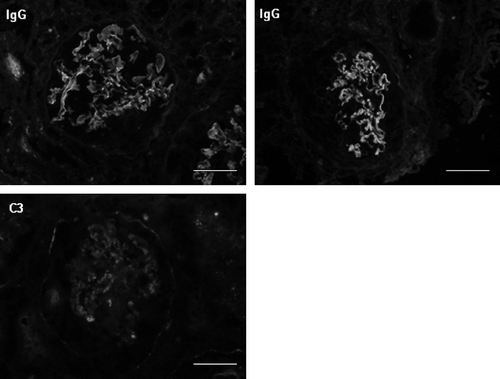
On the 11th day of hospitalization, we performed renal biopsy to elucidate the etiology of RPGN. Light microscopic findings of biopsy specimens revealed cellular crescent formation in 13 of 17 glomeruli (A). The remaining glomeruli showed mild mesangial proliferation. Destruction of Bowman’s capsule (, arrows) and GBM (A, arrow head) was also observed in some glomeruli. The severity of tubular atrophy, interstitial cell infiltration, and fibrosis was intermediated. Neither small vessel angiitis nor granulomas were observed in the interstitium. Immunostaining with anti-IgG antibody, anti-C3 antibody, and anti-fibrin antibody showed linear staining along the glomerular capillary walls (). Electron microscopy showed no electron-dense deposits in glomeruli. Renal biopsy findings indicated anti-GBM antibody glomerulonephritis.
Figure 3. (A) Periodic acid–methenamine silver stain, glomeruli showing cellular crescent. (B) Periodic acid–methenamine silver stain, glomerulus showing destruction of Bowman’s capsule (arrows) and GBM (arrow head).
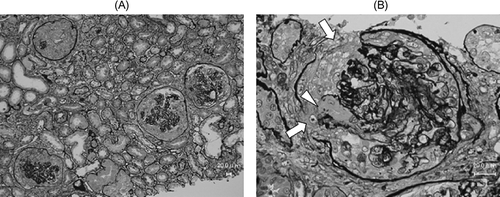
Figure 4. Immunofluorescence with anti-IgG antibody and anti-C3 antibody: capillary linear pattern. IgA, IgM, C4, and C1q were negative.
Note: Scale bars = 50 μm.
After pulmonary infective diseases were ruled out, pulse intravenous methylprednisolone (1 g/day for 3 days) was given. An oral dose of 50 mg of cyclophosphamide was also started. During the clinical course, the patient did not develop hemoptysis and renal failure ameliorated. After the 25th day of hospitalization, both anti-GBM antibodies and MPO-ANCA fell to within the normal range. On chest CT examination, the multiple nodules in the lung disappeared. Consequently, prednisolone was tapered to a dose of 20 mg by 6 weeks, and cyclophosphamide was switched to azathioprine by 4 weeks. No adverse effects of treatment were apparent. Within 3 months of the initiation of treatment, urinary findings were also normalized.
DISCUSSION
Poor renal prognosis in anti-GBM antibody diseases remains a serious clinical problem, despite the fact that patient survival has improved. In previous reports, the following factors predicted poor prognosis: (1) serum creatinine more than 500–600 μmol/L (5.7–6.6 mg/dL) on diagnosis;Citation2–4,11–13 (2) crescent formation more than 50–80%;2,3,12 (3) immediate requirement of dialysis;3 and (4) oliguria or anuria on diagnosis.Citation2,11,14 On admission, our patient presented with a creatinine level of 5.96 mg/dL and required hemodialysis within 72 h. Pathological findings revealed that the crescent formation rate was 76.5%. According to previous reports, these findings indicate poor renal prognosis. However, the patient responded to treatment and achieved remission of glomerulonephritis. The patient’s long-term renal prognosis is expected to be good, unless she develops recurrent glomerulonephritis.
The pathogenicity of anti-GBM antibodies has been demonstrated by passive transfer experiments.Citation7,15 The target autoantigen of anti-GBM antibody has been identified as the noncollagen domain 1 of the alpha3 chain of type IV collagen [α3 (IV)NC1]. Clinically, a rapid reduction in anti-GBM antibody levels appears to be necessary, but such reduction cannot be achieved by drug therapy alone. Therefore, treatment with plasmapheresis combined with corticosteroids and cyclophosphamide is usually utilized.Citation1–3,16,17 In our patient, we consider that plasma exchange was predominant in reducing anti-GBM antibody level, even though corticosteroids were also required. Pusey et al.Citation3,18 emphasized that intensive plasma exchange with 4 L of replacement fluid combined with prednisolone and cyclophosphamide is generally successful in reducing anti-GBM antibody level. Moreover, recent reports have suggested that, in addition to B-cell function, autoreactive T cells may play a causative role in glomerulonephritis.Citation19,20 In our case, a more intensive plasma exchange with 3.6 L of fresh frozen plasma, corresponding to about twice as much as the originally estimated plasma volume, might have contributed to the prompt reduction of anti-GBM antibody level and some inflammatory mediators.
Our patient presented double-positive for anti-GBM antibody and MPO-ANCA. Patients with ANCA-positive anti-GBM antibody disease have some clinicopathological data that suggest an association with systemic vasculitis.Citation4,6–8 Renal pathological findings in this case did not show evident ANCA-associated vasculitis-like findings such as small vessel angiitis or granulomas, but she was suspected to have pulmonary involvement of ANCA-associated vasculitis. ANCA-positive patients tend to have lower anti-GBM antibody titers than ANCA-negative patients.Citation4,6 Intriguingly, according to previous reports, renal prognosis of patients with double-positive antibodies is varied. Some older reports suggested that renal prognosis in double-positive patients is better than that in patients with anti-GBM antibody alone.Citation6,9,10 They concluded that higher titers of ANCA and lower titers of anti-GBM antibody might contribute to better renal prognosis of double-positive patients, because clinical properties of such patients are not similar to those of anti-GBM antibody disease, but to those of systemic vasculitis.
In contrast, Levy et al.Citation4 reported that renal prognosis is poorer in double-positive patients than in patients who are positive for only anti-GBM antibody. They examined the specificity of anti-GBM antibodies and excluded patients with anti-GBM antibody bound to other components of GBM, but not to α3(IV)NC1. They concluded that renal prognosis might be better in double-positive patients with anti-GBM antibody not specific to α3(IV)NC1. Hellmark et al.Citation21 reported that patients with anti-GBM antibody disease exhibit an inverse relationship between renal survival and the titers of autoantibody specific to the N-terminus of α3(IV)NC1. Yang et al.Citation8 reported that double-positive patients had lower levels of antibodies against α3(IV)NC1 compared with those of patients with anti-GBM antibody alone. In addition, patients with high levels of anti-GBM antibodies against specific epitopes on α3(IV)NC1 have an even worse prognosis in anti-GBM antibody disease.Citation22 Given these findings, there might be heterogeneity in clinical characteristics of anti-GBM antibody disease on the basis of the titers of anti-GBM antibodies specific for α3(IV)NC1. In our case, anti-GBM antibody titers were low (38 EU), although renal findings were severe and specific for anti-GBM antibody-mediated glomerulonephritis with marked cellular crescents. The low titers, and perhaps the low specificity, in the presence of MPO-ANCA might have led to the patient’s good clinical course.
Intensive immunosuppressive therapy has potentially serious complications, such as sepsis or Pneumocystis jiroveci pneumonia.Citation4,5 Therefore, in the guidelines for anti-GBM antibody disease published by the Japanese Society of Nephrology, intensive combination therapy is not indicated for patients with a creatinine concentration exceeding 6.0 mg/dL or for those with crescent formation of more than 50%. We initiated treatment with plasma exchange and prednisolone, and then administered cyclophosphamide after pulmonary infective diseases were ruled out. Accordingly, the patient developed no adverse effects. If further prognostic factors can be identified, we could further improve patient and renal survival by providing more adequate treatment than at present.
To conclude, we reported a case of MPO-ANCA-positive anti-GBM antibody disease, in which anti-GBM antibody normalization and dialysis independence were achieved within 3 weeks, without complication, by treatment with plasma exchange and prednisolone. The most critical determinant of renal prognosis remains early treatment. Furthermore, in patients with MPO-ANCA-positive anti-GBM antibody disease, intensive therapy predominantly with plasma exchange might be effective, even though renal function is less likely to recover.
ACKNOWLEDGMENTS
We give our thanks to Hisatsugu Goto, Momoyo Azuma, Ryo Tobiume (Department of Medicine and Rheumatology, Institute of Health Bioscience, The University of Tokushima Graduate School), and Michael Hann (Florida International University) for technical assistance.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Lockwood CM, Rees AJ, Pearson TA, Evans DJ, Peters DK, Wilson CB. Immunosuppression and plasma-exchange in the treatment of Goodpasture’s syndrome. Lancet. 1976;1:711–715.
- Bolton WK. Goodpasture’s syndrome. Kidney Int. 1996;50:1753–1766.
- Levy JB, Turner AN, Rees AJ, Pusey CD. Long-term outcome of anti-glomerular basement membrane antibody disease treated with plasma exchange and immunosuppression. Ann Intern Med. 2001;134:1033–1042.
- Levy JB, Hammad T, Coulthart A, Dougan T, Pusey CD. Clinical features and outcome of patients with both ANCA and anti-GBM antibodies. Kidney Int. 2004;66:1535–1540.
- Hirayama K, Yamagata K, Kobayashi M, Koyama A. Anti-glomerular basement membrane antibody disease in Japan: Part of the nationwide rapidly progressive glomerulonephritis survey in Japan. Clin Exp Nephrol. 2008;12:339–347.
- Jayne DR, Marshall PD, Jones SJ, Lockwood CM. Autoantibodies to GBM and neutrophil cytoplasm in rapidly progressive glomerulonephritis. Kidney Int. 1990;37:965–970.
- Rutgers A, Meyers KE, Canziani G, Kalluri R, Lin J, Madaio MP. High affinity of anti-GBM antibodies from Goodpasture and transplanted Alport patients to alpha3(IV)NC1 collagen. Kidney Int. 2000;58:115–122.
- Yang R, Hellmark T, Zhao J, . Antigen and epitope specificity of anti-glomerular basement membrane antibodies in patients with Goodpasture disease with or without anti-neutrophil cytoplasmic antibodies. J Am Soc Nephrol. 2007;18:1338–1343.
- Bosch X, Mirapeix E, Font J, . Prognostic implication of anti-neutrophil cytoplasmic autoantibodies with myeloperoxidase specificity in anti-glomerular basement membrane disease. Clin Nephrol. 1991;36:107–113.
- Segelmark M, Hellmark T, Wieslander J. The prognostic significance in Goodpasture’s disease of specificity, titre and affinity of anti-glomerular-basement-membrane antibodies. Nephron Clin Pract. 2003;94:c59–c68.
- Cui Z, Zhao MH, Xin G, Wang HY. Characteristics and prognosis of Chinese patients with anti-glomerular basement membrane disease. Nephron Clin Pract. 2005;99:c49–c55.
- Merkel F, Pullig O, Marx M, Netzer KO, Weber M. Course and prognosis of anti-basement membrane antibody (anti-BM-Ab)-mediated disease: Report of 35 cases. Nephrol Dial Transplant. 1994;9:372–376.
- Daly C, Conlon PJ, Medwar W, Walshe JJ. Characteristics and outcome of anti-glomerular basement membrane disease: A single-center experience. Ren Fail. 1996;18:105–112.
- Walker RG, Scheinkestel C, Becker GJ, Owen JE, Dowling JP, Kincaid-Smith P. Clinical and morphological aspects of the management of crescentic anti-glomerular basement membrane antibody (anti-GBM) nephritis/Goodpasture’s syndrome. Q J Med. 1985;54:75–89.
- Lerner RA, Glassock RJ, Dixon FJ. The role of anti-glomerular basement membrane antibody in the pathogenesis of human glomerulonephritis. J Exp Med. 1967;126:989–1004.
- Johnson JP, Moore J, Jr, Austin HA, III, Balow JE, Antonovych TT, Wilson CB. Therapy of anti-glomerular basement membrane antibody disease: Analysis of prognostic significance of clinical, pathologic and treatment factors. Medicine (Baltimore). 1985;64:219–227.
- Couser WG. Rapidly progressive glomerulonephritis: Classification, pathogenetic mechanisms, and therapy. Am J Kidney Dis. 1988;11:449–464.
- Pusey CD. Anti-glomerular basement membrane disease. Kidney Int. 2003;64:1535–1550.
- Salama AD, Chaudhry AN, Holthaus KA, . Regulation by CD25+ lymphocytes of autoantigen-specific T-cell responses in Goodpasture’s (anti-GBM) disease. Kidney Int. 2003;64:1685–1694.
- Wolf D, Hochegger K, Wolf AM, CD4+CD25+ regulatory T cells inhibit experimental anti-glomerular basement membrane glomerulonephritis in mice. J Am Soc Nephrol. 2005;16:1360–1370.
- Hellmark T, Segelmark M, Unger C, Burkhardt H, Saus J, Wieslander J. Identification of a clinically relevant immunodominant region of collagen IV in Goodpasture disease. Kidney Int. 1999;55:936–944.
- Yang R, Hellmark T, Zhao J, . Levels of epitope-specific autoantibodies correlate with renal damage in anti-GBM disease. Nephrol Dial Transplant. 2009;24:1838–1844.
