Abstract
Background: Immunoglobulin A (IgA) nephropathy is the most common cause of primary glomerulonephritis with slow progression to end-stage renal disease (ESRD) in up to 40% of patients. Methods: A retrospective cohort study of patients with biopsy-proven IgA nephropathy was performed in our center from 1998 to 2009. We tried to determine the clinical and pathological factors which affect patients progressing to ESRD. We also compared the impact of renin–angiotensin system (RAS) blockers therapy alone or in combination with prednisone on baseline proteinuria and glomerular filtration rate (GFR) after 6 months of treatment in patients with proteinuria>1 g/d and GFR>30 mL/min. Results: There were 70 IgA nephropathy patients of whom 46 were men. The average age of patients at biopsy was 39 ± 12.1 years. During the median 23.5 (6–130) months of follow-up, 10 patients progressed to ESRD and no patient died. Five-year renal survival following biopsy was 88%. By multivariate analysis, age more than 50 years (p = 0.003) and baseline serum creatinine level (p = 0.012) were independent predictors of progressing to ESRD and poor prognosis. Although there was no significant difference in proteinuria reduction after 6 months of treatment, kidney function was less preserved in RAS inhibitors therapy alone than in the combination treatment with prednisone. Conclusion: We showed that late diagnosis of patients with IgA nephropathy might be associated with poor outcome. Our results also suggest that addition of prednisone to RAS blockers may lead to better preservation of kidney function.
INTRODUCTION
IgA nephropathy is the most common form of glomerulonephritis in the world.Citation1 This disease is characterized by recurrent episodes of macroscopic hematuria or asymptomatic persistent microscopic hematuria with or without proteinuria.Citation2,3 Studies indicated that up to 40% of IgA nephropathy patients eventually would develop end-stage renal disease (ESRD).Citation4–6 Many studies have tried to identify the prognostic factors of IgA nephropathy including male sex, elevated serum creatinine, nephrotic range proteinuria, hypertension, and high histological grade.Citation6–8 However, the prognosis is quite variable and the outcome is difficult to predict with accuracy in individual patients.Citation9 In this study, we determined the prognostic indicators of progression to ESRD in our IgA nephropathy patients. We also investigated whether combination therapy with prednisone and renin–angiotensin system (RAS) blockers was superior to RAS inhibitors therapy alone in preserving the kidney function of patients with proteinuria >1 g/24 h and glomerular filtration rate (GFR) > 30 mL/min/1.73 m2.
PATIENTS AND METHODS
All patients with the histological diagnosis of IgA nephropathy in Dr. Shariati Hospitalâ from July 1998 to March 2009 were reviewed. Only the patients who had been followed for at least six months after kidney biopsy and did not suffer from systemic diseases, were enrolled in our study. The duration of follow-up was defined as period of time up to ESRD (need for renal replacement therapy) or the last visit. Baseline clinical and demographic data at the time of renal biopsy were collected. These included sex, age, presence of hypertension [defined as mean arterial pressure (MAP) ≥ 107], serum creatinine level, and 24 h urine protein. The number of ESRD events during follow-up and the duration from renal biopsy to ESRD was recorded. In our center, main reasons for performing a renal biopsy were persistent microscopic hematuria and proteinuria more than 0.5 g/d, daily proteinuria more than 1.5 g/24 h, and renal impairment of unknown origin. The diagnosis of IgA nephropathy was made by the detection of mesangial deposits of IgA in immunofluorescence staining. We reviewed all renal biopsies to identify their appropriate grades. The biopsies were classified into grades I, II, or III in increasing order of severity according to a three-grade histological classification.Citation10 Grade I indicated the presence of normal glomeruli or slight increase in mesangial matrix and/or cellularity without tubulointerstitial changes. Grade II denoted a moderate degree of mesangial proliferation and/or focal segmental sclerosis and/or cellular crescents up to 50% of glomeruli, and tubule atrophy and interstitial fibrosis up to 1/3 of cortical area. Grade III indicated cellular crescents in more than 50% of glomeruli and/or global glomerulosclerosis or fibrous crescents involving >1/3 of glomeruli, and tubulointerstitial changes involving more than 1/3 of cortical area.
The minimum follow-up for patients was 6 months, and we also assessed the impact of treatment on baseline proteinuria and GFR during that time. For this purpose, first we calculated baseline and 6-month estimated GFR (by Modification in Diet and Renal Disease equation). Then patients with baseline GFR > 30 mL/min/1.73 m2 and baseline proteinuria > 1 g/24 h and on RAS inhibitors therapy alone [angiotensin-converting enzyme inhibitors (ACEIs) and/or angiotensin receptor blockers (ARBs) or in combination with prednisone (0.8–1 mg/kg/d at baseline with tapering off during 6 months) were enrolled (43 patients). Finally, we compared baseline, 6-month, and delta (differences between 6-month and baseline values) proteinuria and GFR between the two groups (RAS inhibitors alone or in combination with prednisone) if baseline proteinuria was between 1 and 3 g/24 h or more than 3 g/24 h.
Statistics
Continuous variables were expressed as mean ± SD or median and interquartile range, and categorical data were expressed as frequencies and percentages. The differences between groups were compared with Student t-test or Mann–Whitney U test as appropriate. For comparison of continuous data between more than two groups, we used ANOVA and Scheffe test as post hoc analysis. Categorical variables were compared with chi-square test. Renal survival was estimated by the Kaplan–Meier method. We used univariate and multivariate binary logistic regression analysis to assess risk factors of ESRD. For therapy assessment, due to non-Gaussian distribution, continuous data were expressed as median (minimum, maximum) and were compared using Mann–Whitney U test, and categorical data were compared by chi-square test or Fisher’s exact test when appropriate.
RESULTS
Baseline demographic and clinical findings at the time of renal biopsy are listed in . There were 70 IgA nephropathy patients of whom 46 were men (male to female ratio 1.9:1). The average age at biopsy was 39 ± 12.1 (range 17–71) years. The median duration of follow-up was 23.5 (6–130) months. Arterial hypertension was present in 25 (35.7%) of patients. At renal biopsy, median serum creatinine level was 1.6 mg/dL (interquartile range, 1–2.1), median daily proteinuria was 1829 g/24 h (interquartile range, 989–3611), and average MAP was 103.6 ± 14.0 mmHg. All patients except one (because of hyperkalemia) were treated with ACEI and/or ARBs. The distribution of histological lesions was as follows: 12 patients (17.1%) grade I, 38 subjects (54.3%) grade II, and 20 patients (28.6%) grade III. There were significant differences among groups (grade I the best and grade III the worst) for age (grade III vs. I, p = 0.033), number of patients with hypertension (p = 0.009), median serum creatinine level (p < 0.001), median daily proteinuria (p = 0.002), and number of ESRD (p < 0.001).
Table 1. Demographic and clinical characteristics of patients with IgA nephropathy.
Renal Survival
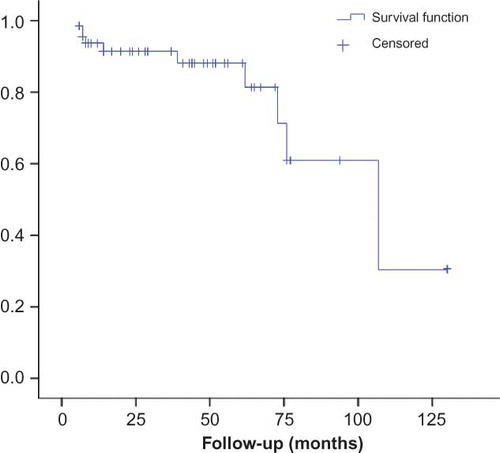
During follow-up period, 10 ESRD events happened (). Median time of progression to ESRD was 107 months (CI: 95% 62–152). One-year kidney survival following biopsy was 94%, 3-year was 91%, and 5-year was 88%. No patient died before ESRD during the follow-up period. The Kaplan–Meier survival curve is shown in . ESRD events were associated with older age (>50 years, p = 0.001), higher pathological grading (p < 0.001), greater baseline serum creatinine level (p = 0.011), and more daily proteinuria (p = 0.004). In univariate analysis, odds ratio of ESRD events for patients older than 50 years was 13.5 (CI: 95% 2.9–61.7, p = 0.001), for every unit increase in serum creatinine (mg/dL) was 3.7 (1.6–8.6, p = 0.003) and for daily proteinuria more than 3 g/24 h was 13.1 (2.5–69.2, p = 0.002). We had no ESRD in patients with grade I pathology, and odds ratio of ESRD event for grade III versus II was 12 (2.2–64.5, p = 0.004). ESRD events also were more common in male and hypertensive patients but not at a significant level. In multivariate analysis, age older than 50 years (odds ratio 16.3, CI 2.6–102.8, p = 0.003) and baseline serum creatinine level (odds ratio 3.8, CI 1.3–10.9, p = 0.012) were independent predictors of poor outcome.
Table 2. Demographic and clinical characteristics of IgA nephropathy patients with and without ESRD.
Therapy Assessment
Results of therapy analysis are shown in . There were no significant differences in age, sex, frequency of hypertension, or pathological grade, and median baseline, 6-month, delta proteinuria in RAS inhibitors therapy alone group and in combination therapy with prednisone group, whether baseline proteinuria was 1–3 g/24 h or more than 3 g/24 h.
Table 3. Comparison of changes in proteinuria and GFR after 6 months of treatment with RAS inhibitors or combination therapy with prednisone.
In patients with baseline proteinuria of 1–3 g/24 h, median baseline GFR in RAS inhibitors group was 75.3 (54–137) and in combination group was 50.2 (30.1, 105.3) with p = 0.003. Median 6-month GFR in RAS inhibitors group and combination group respectively were 66.8 (58.7, 105.2) and 60.9 (29.4, 135.5) with p = 0.043. There was no significant difference (p = 0.067) in median delta GFR between RAS inhibitors group (−7.1; −31.8, 46.9) and combination group (7.4; −53.1, 78.8).
In patients with baseline proteinuria > 3 g/24 h, there was no significant difference in median baseline and 6-month GFR between two groups. Median delta GFR was significantly higher in combination group (12.7; −47.5, 49.6) versus RAS inhibitors group (−2.2; −38.6, 0); p = 0.055.
DISCUSSION
IgA nephropathy is the most common primary glomerulonephritis in the world today.Citation1 Although initially thought to be a benign disease, it is progressively recognized as a cause of ESRD in a significant number of patients within 10–20 years from onset of disease.Citation11
The identification of clinical and histological factors at renal biopsy that affect disease progression in patient with IgA nephropathy has stimulated a great deal of research for years.Citation6,9,12–35 They reported predictor risk factors and somehow different renal survival based on whether screening programs were practiced in their countries.Citation28,33,35–38 However, variability in disease severity should be considered as well.Citation35 It is important to understand which patients are at risk for progression to ESRD and who benefit from more aggressive therapeutic interventions.
In this study, we reviewed the clinical features and outcome of a cohort of primary IgA nephropathy patients. Five-year renal survival following biopsy was 88%. Most of our patients were biopsied for diagnosis at a relatively late stage with 60% of patients classified in chronic kidney disease stage III and higher. This indicates that our patients were often referred to nephrologists for renal biopsy at a late stage. In a study on 204 Chinese patients who were biopsied at an early stage of disease, 5-year survival following biopsy was 85.1% that is lower than our report despite the late referral of our patients.Citation38
Almost 31% of our patients showed urine protein excretion greater than 3 g/d, 30% had serum creatinine level more than 2 mg/dL, and 28.6% revealed severe pathological involvements at time of biopsy.
Although hypertension at the time of diagnosis was prevalent in our subjects (35.7%), hypertension did not affect renal prognosis which might be explained by prescribing RAS inhibitors in all patients except one. Likewise, treatment with RAS inhibitors with or without prednisone was associated with a considerable reduction in proteinuria; this may explain the lack of independent effect of the baseline proteinuria on poor outcome.
Histological grading did not reveal independent impact on renal survival; however, more than 50% of patients with grade III pathological diagnosis had baseline estimated GFR less than 30 mL/min/1.73 m2.
Multivariate analysis indicated that baseline serum creatinine level and age greater than 50 years were independent predictors of renal survival. Six of 10 patients who reached ESRD were more than 50 years old and their GFR at biopsy was less than 60 mL/min/1.73 m2. It is possible that poor prognosis in older patients occurs because of late diagnosis rather than the more aggressive course, though disease severity cannot be ruled out.
Most studies have shown that glucocorticoid therapy in IgA nephropathy whenever indicated could lead to both a reduction in proteinuria and perhaps improved renal survival in individuals with preserved kidney functionCitation39–42 and those with more advanced stage of disease.Citation43–45 In specific conditions, combination therapy with steroid plus RAS inhibitors may be superior to RAS inhibitors therapy alone.Citation45,46
Lv et al.Citation45 in a randomized control trail of 63 IgA nephropathy patients with proteinuria 1–5 g/d and GFR > 30 mL/min/1.73 m2 and follow-up for up to 48 months found that combination therapy with cilazapril and prednisone was associated with better outcome for kidney function and more rapid decrease in proteinuria than cilazapril therapy alone. In a multicenter study by Manno et al,Citation46 combined treatment with ramipril and a 6 months course of prednisone suggested greater benefit in preventing progression of kidney disease compared with ramipril alone in 97 IgA nephropathic patients with proteinuria > 1 g/d and GFR > 50 mL/min/1.73 m2 with a 8-year follow-up. In this study monotherapy was associated with a poor outcome and slow reduction in proteinuria.
Our study indicated that in patients with moderately severe IgA nephropathy (proteinuria more than 1 g/d and mild to moderate decrease in renal function), in terms of maintaining or improving kidney function at least in the short term, combination therapy with prednisone was superior to RAS inhibitors therapy alone; this effect probably is independent of proteinuria reduction because both treatments led to almost the same decrease in proteinuria. Therefore, combination treatment with RAS inhibitors and prednisone might present a better choice in treatment of patients with moderately severe disease.
In summary, IgA nephropathy is not benign nephritis, and slow progression to ESRD could occur. However, early diagnosis and timely intervention may improve the renal prognosis. Older age and baseline serum creatinine level could independently affect renal survival. Our results also suggest that addition of prednisone to RAS blockers in moderately severe disease at least in short term may lead to better preservation of kidney function. However, this is a retrospective study with small number of patients and relatively short follow-up. Also, we evaluated only the impact of treatment in 6-month duration, and rate of proteinuria reduction was not assessed.
ACKNOWLEDGMENT
The authors thank the Research and Development Center of Shariati Hospital for helping on the statistical aspects of this manuscript.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- D’Amico G. The commonest glomerulonephritis in the world: IgA nephropathy. Q J Med. 1987;64:709–727.
- Schena FP. A retrospective analysis of the natural history of primary IgA nephropathy worldwide. Am J Med. 1990;89:209–215.
- Coppo R, Amore A, Hogg R, Emancipator S. Idiopathic nephropathy with IgA deposits. Pediatr Nephrol. 2000;15:139–150.
- Clarksson AR, Woodroffe AJ, Aarons I. IgA nephropathy in patients followed-up for at least ten years. Semin Nephrol. 1987;7:377–378.
- D’Amico G, Colasanti G, di Belgioioso GB, et al. Long term follow up of IgA mesangial nephropathy: Clinico-histological study in 374 patients. Semin Nephrol. 1987;7:355–358.
- Johnston PA, Brown JS, Braumholtz DA, Davison AM. Clinico-pathological correlations and long-term follow-up of 253 United Kingdom patients with IgA nephropathy. A report from the MRC Glomerulonephritis Registry. Q J Med. 1992;84:619–627.
- Ygame M, Suzuki D, Jiade K, . Value of pathological grading in prediction of renal survival in IgA nephropathy. Nephrology. 1996;2:107–117.
- D’Amico G. Natural history of idiopathic IgA nephropathy and factors predictive of disease outcome. Semin Nephrol. 2004;24:179–196.
- Beukhof JR, Kardaun O, Schaafsma W, . Toward individual prognosis of IgA nephropathy. Kidney Int. 1986;29:549–556.
- Manno C, Giovanni F, Strippoli M, Torres D, Rossini M, Schena FP. A novel simpler histological classification for renal survival in IgA nephropathy: A retrospective study. Am J Kid Dis. 2007;49:763–775.
- Julian BA, Waldo FB, Rifai A, Mestecky J. IgA nephropathy, the most common glomerulonephritis world-wide: A neglected disease in the United States? Am J Med. 1988;84:129–132.
- Nicholls KM, Fairly KF, Dowling JP, Kincaid-Smith P. The clinical course of mesangial IgA associated nephropathy in adults. Q J Med. 1984;53:22–50.
- D’Amico G, Minetti L, Ponticelli G, . Prognostic indicators in idiopathic IgA mesangial nephropathy. Q J Med. 1986;59:363–378.
- Woo KT, Edmondson RPS, Wu AYT, . The natural history of IgA nephritis in Singapore. Clin Nephrol. 1986;25:15–21.
- Kusumoto Y, Takebayashi S, Taguchi T, Harada T, Naito S. Long-term prognosis and prognostic indices of IgA nephropathy in juvenile and adult Japanese. Clin Nephrol. 1987;28:118–124.
- Velo M, Lozano L, Egido J, Gutierrez-Millet V, Hernando L. Natural history of IgA nephropathy in patients followed up for more than ten years in Spain. Semin Nephrol. 1987;7:346–350.
- Bogenschutz O, Bohle A, Batz C, . IgA nephritis: On the importance of morphological and clinical parameters in the long-term prognosis of 239 patients. Am J Nephrol. 1990;10:137–147.
- Rekola S, Bergstrand A, Buch H. IgA nephropathy. Development of hypertension in IgA nephropathy as a marker of poor prognosis. Am J Nephrol. 1990;10:290–295.
- Alamartine E, Sabatier JC, Guerin C, Berliet JM, Berthoux F. Prognostic factors in mesangial IgA glomerulonephritis: An extensive study with univariate and multivariate analyses. Am J Kidney Dis. 1991;18:12–19.
- Ibels LS, Gyory AZ. IgA nephropathy: Analysis of the natural history, important factors in the progression of renal disease, and a review of the literature. Medicine. 1994;73:79–109.
- Li T, Ho L, Szeto C, Yu LyMee, Lai M-M. Prognostic indicators of IgA nephropathy in the Chinese-clinical and pathological perspectives. Nephrol Dial Transplant. 2002;17:64–69.
- D’Amico G. Influence of clinical and histological features on actuarial renal survival in adult patients with idiopathic IgA nephropathy, membranous nephropathy, and membranoproliferative glomerulonephritis: Survey of the recent literature. Am J Kidney Dis. 1992;20:315.
- Szeto CC, Lai F, To KF, . The natural history of immunoglobulin A nephropathy among patients with hematuria and minimal proteinuria. Am J Med. 2001;110:434.
- D’Amico G. Natural history of idiopathic IgA nephropathy: Role of clinical and histological prognostic factors. Am J Kidney Dis. 2000;36:227.
- Rekola S, Bergstrand A, Bucht H. Deterioration of GFR in IgA nephropathy as measured by 51Cr-EDTA clearance. Kidney Int. 1991;40:1050.
- Nozawa R, Suzuki J, Takahashi A, . Clinicopathological features and the prognosis of IgA nephropathy in Japanese children on long-term observation. Clin Nephrol. 2005;64:171.
- Lau KK, Gaber LW, Delos Santos NM, Fisher KA, Grimes SJ, Wyatt RJ. Pediatric IgA nephropathy: Clinical features at presentation and outcome for African-Americans and Caucasians. Clin Nephrol. 2004;62:167.
- Bartosik LP, Lajoie G, Sugar L, Cattran DC. Predicting progression in IgA nephropathy. Am J Kidney Dis. 2001;38:728.
- Hall CL, Bradley R, Kerr A, Attoti R, Peat D. Clinical value of renal biopsy in patients with asymptomatic microscopic hematuria with and without low-grade proteinuria. Clin Nephrol. 2004;62:267.
- Ikee R, Kobayashi S, Saigusa T, . Impact of hypertension and hypertension-related vascular lesions in IgA nephropathy. Hypertens Res. 2006;29:15.
- Donadio JV, Bergstralh EJ, Grande JP, Rademcher DM. Proteinuria patterns and their association with subsequent end-stage renal disease in IgA nephropathy. Nephrol Dial Transplant. 2002;17:1197.
- Reich HN, Troyanov S, Scholey JW, Cattran DC. Remission of proteinuria improves prognosis in IgA nephropathy. J Am Soc Nephrol. 2007;18:3177.
- Haas M. Histologic subclassification of IgA nephropathy: A clinicopathologic study of 244 cases. Am J Kidney Dis. 1997;29:829.
- Lee HS, Lee MS, Lee SM, . Histological grading of IgA nephropathy predicting renal outcome: Revisiting H. S. Lee’s glomerular grading system. Nephrol Dial Transplant. 2005;20:342.
- Geddes CC, Rauta V, Gronhagen-Riska C, . A tricontinental view of IgA nephropathy. Nephrol Dial Transplant. 2003;18:1541.
- Radford MG, Donadio JV, Bergstralh EJ, Grande JP. Predicting renal outcome in IgA nephropathy. J Am Soc Nephrol. 1997;8:199–207.
- Schena FP. Immunoglobulin A nephropathy with mild renal lesions: A call in the forest for physicians and nephrologists. Am J Med. 2001;110:499–500.
- Lv J, Zhang H, Zhou Y, Li G, Zou W, Wang H. Natural history of immunoglobulin A nephropathy and predictive factors of prognosis: A long-term follow up of 204 cases in China. Nephrology. 2008;13:242–246.
- Kobayashi Y, Hiki Y, Fujii K, Kurokawa A, Tateno S. Moderately proteinuric IgA nephropathy: Prognostic prediction of individual clinical courses and steroid therapy in progressive cases. Nephron. 1989;53:250.
- Kobayashi Y, Hiki Y, Kokubo T, Horii A, Tateno S. Steroid therapy during the early stage of progressive IgA nephropathy. A 10-year follow-up study. Nephron. 1996;72:237.
- Pozzi C, Bolasco PG, Fogazzi GB, . Corticosteroids in IgA nephropathy: A randomised controlled trial. Lancet. 1999;353:883.
- Pozzi C, Andrulli S, Del Vecchio L, Melis P. Corticosteroid effectiveness in IgA nephropathy: Long-term results of a randomized, controlled trial. J Am Soc Nephrol. 2004;15:157.
- Tamura S, Ueki K, Ideura H, . Corticosteroid therapy in patients with IgA nephropathy and impaired renal function. Clin Nephrol. 2001;55:192.
- Moriyama T, Honda K, Nitta K, Yumura W, Nihei H. The effectiveness of steroid therapy for patients with advanced IgA nephropathy and impaired renal function. Clin Exp Nephrol. 2004;8:237.
- Lv J, Zhang H, Chen Y, . Combination therapy of prednisone and ACE inhibitor versus ACE-inhibitor therapy alone in patients with IgA nephropathy: A randomized controlled trial. Am J Kidney Dis. 2009;53:26.
- Manno C, Torres DD, Rossini M, Pesce F, Schena FP. Randomized controlled clinical trial of corticosteroids plus ACE-inhibitors with long-term follow-up in proteinuric IgA nephropathy. Nephrol Dial Transplant. 2009;24:3694.