Abstract
Background and aims: The aim of this study was to investigate the possible influence of tumor necrosis factor-α (TNF-α) gene promoter polymorphisms on the hepatitis C virus (HCV) infection in Chinese hemodialysis (HD) patients. Methods: A total of 884 HD patients from 14 HD centers in south China were investigated. The TNF-α gene promoter polymorphisms at positions 238 and 308 of the patients were detected by the polymerase chain reaction (PCR)–restriction fragment length polymorphism method. Results: Among the 884 patients, 176 (19.9%) were anti-HCV (+), and 142 (80.7%) of the anti-HCV (+) patients became chronically infected. The anti-HCV (+) patients showed longer duration of HD, higher rate of blood transfusion, kidney transplantation, and dialyzer reuse, compared with the anti-HCV (−) patients. However, the distributions of TNF-α −238 and −308 alleles and genotypes had no significant differences between the anti-HCV (+) and the anti-HCV (−) patients (p > 0.05). And the frequencies of the above alleles and genotypes were also approximately equally distributed in the persistent infection and in the viral clearance HD patients (p > 0.05). Conclusions: This study did not suggest that the TNF-α −238 and −308 polymorphisms had influence on the infection of HCV in Chinese HD patients.
INTRODUCTION
Hepatitis C virus (HCV) has been recognized as the major cause of acute and chronic hepatitis among patients with end-stage renal disease undergoing hemodialysis (HD), although the prevalence of the infection differs among countries and regions, ranging from 7% to 40%.Citation1–3 The mortality rate increased in the HD patients with hepatitis C.Citation4–6 The natural outcome of HCV infection varies dramatically among individuals, including spontaneous clearance in a minority and persistent infection for the most patients, which may progress to cirrhosis and eventually hepatocellular carcinoma.Citation7,8 Although the determinants of susceptibility to infection, self-limiting, or development of chronicity have not been well defined, many investigators have drawn attention to the significance of immunity-related genes.
From different lines of evidence, Th1 and Th2 responses have been shown to be interacted, and it seemed that a shift in the balance between Th1 and Th2 cytokines levels may contribute to spontaneous virus clearance, fibrosis, and response to therapy.Citation9–11 The key roles of the cytokines in the pathogenesis and the self-limiting of HCV infection are becoming clearer. Tumor necrosis factor-α (TNF-α), one of the most important Th1 cytokines, exhibits a wide range of biological properties, including polarization of a Th1 response, immune response to infectious agents, and direct antiviral effects, is important in the host defense against viruses.
Cytokine gene sequences are polymorphic at specific sites, and some mutations located within the coding and regulatory regions could powerfully control the production of specific cytokines.Citation12–14 The TNF-α gene is located on chromosome 6, in the class III region of the major histocompatibility complex between HLA-B and HLA-DR. In the TNF-α promoter gene, the presence of G or A nucleotide at position −238 and −308 generated three potential genotypes GG, GA, and AA, which may correspond phenotypically to the production of different levels of TNF-α.Citation13,14 Several studies have investigated the association between TNF-α gene promoter variants and the susceptibility to HCV infection, development of acute viral hepatitis, spontaneous clearance of HCV infection, and persistent infection in general population,Citation15–18 but the conclusions were inconsistent. Few investigators have described the effect of TNF-α gene polymorphisms in HD patients infected with HCV.Citation15–18 As we know, the incidence of HCV infection is high in the HD units, and the patients infected with HCV were under multiple influences, such as chronic uremia status and impaired immune response, which were significantly different from the general population. The fact above prompted us to investigate the possible associations between TNF-α polymorphisms and HCV infection in Chinese HD patients.
SUBJECTS AND METHODS
Patients
A total of 884 HD patients from 14 HD centers in south of China were enrolled in this study, which included 556 males and 328 females. The mean age was 52.62 ± 13.44 years and the mean duration of HD was 3.56 ± 3.81 years. They were classified into the two groups: anti-HCV (+) and anti-HCV (−). All the anti-HCV (+) individuals had not taken anti-virus treatment; they were divided into the persistent infection and the viral clearance groups. The persistent infection group was described as positive for anti-HCV antibodies and HCV-RNA for more than 6 months, while the criteria for the viral clearance group were positive for anti-HCV once and having no evidence for being positive for HCV-RNA on at least two occasions 6 months apart. A detailed questionnaire was prepared for these patients to identify general and some clinical information. The Institutional Ethical Committee approved the protocol for sample collection as well as biochemical, viral, and genetic analyses. Informed consent was obtained before enrolling the patients for conducting the various blood tests including studies on genetic polymorphism.
Serology
Five milliliters of blood was collected, and the serum and peripheral blood mononuclear cells were separated and stored in aliquots at −70°C until assay. Liver biochemistry tests including alanine aminotransferase (ALT), aspartate aminotransferase (AST), and γ-glutamyltransferase (GGT) were performed with a HITACHI 7170 automatic biochemistry analyzer. The cut-off levels of AST (<40 and >40), ALT (<40 and >40), and GGT (<50 and >50) were chosen according to literature.Citation19,20
Serum anti-HCV antibodies were detected by the ELISA kit (Shanghai Kehua Biotech Co., Ltd, Shanghai, China). The detection of HCV-RNA was done by diagnostic kit for quantification of HCV-RNA [polymerase chain reaction (PCR)-Fluorescence probing] (Shanghai Kehua Biotech Co., Ltd, Shanghai, China; ABI, California, CA, USA).
Genotyping Assays
Genomic DNA of each subject was extracted from 5 mL of peripheral blood leukocytes using sodium dodecyl sulfate lysis and proteinase K digestion followed by standard phenol–chloroform methods according to standard protocols as previously described.Citation21 After genomic DNA was extracted from the blood samples, the region containing the polymorphic site was amplified by PCR, as reported by van Belzen et al.Citation22 Briefly, PCR was carried out with primers in a 20 μL volume with buffer [10× equals 500 mM KCl, 100 mM Tris-HCl (pH 8.8), 25 mM MgCl2], 1 pM of specific oligonucleotide primers, 100 μM dNTPs, 50 ng of genomic DNA, and 1 U Taq DNA polymerase (Boehringer Mannheim, GmbH, Mannheim, German). PCR was performed using a MJ-PTC-200 Thermal Cycler under the specified PCR conditions. Primer sequences for the gene polymorphism at position −238 were forward 5′-ATCTGGAGGAAGCGGTAGTG-3′ and reverse 5′-AGAAGACCCCCCTCGGAACC-3′, for the polymorphism at −308 forward 5′-AGGCAATAGGTTTTGAGGGCCAT-3′ and reverse 5′-TCCTCCCTGCTCCGATTCCG-3′. The parameters for thermo cycling of the −238 and −308 were the same as follows: 94°C for 6 min, 33 cycles of 94°C for 30 s, 52°C for 30 s, and 72°C for 1 min, there was final 72°C incubation for 10 min.
The amplified PCR products were digested at 37°C with the specified restriction endonuclease MspI (New England Biolabs, Beverly, MA, USA) to detect the single nucleotide polymorphism (SNP) in the −238 gene allele and NcoI (New England Biolabs) to detect the polymorphism of the −308 nucleotide. After digestion, under different conditions recommended by the manufacturer’s instructions, the fragments were electrophoresed on a 3% agarose gel (Biowest Agarose) and stained with ethidium bromide. The sizes of fragments were estimated by comparison with previously known size markers (Tiangen Biotech, Beijing, China).
Genotyping was performed without knowing the subjects’ cases. When genotyping was performed, two research assistants independently read the gel images. If they did not reach a consensus on the tested genotypes (<2%), they repeated the genotyping independently to reach a consensus.
Statistical Analysis
All statistical analyses were performed with SAS and SPSS statistical packages (SAS version 9.13 software packages, SAS Institute, Cary, NC, USA; SPSS version 15.0, Chicago, IL, USA). Multivariate analysis of variance was used to analyze the difference of the biochemical index among patients. The distribution of the TNF-α gene polymorphisms revealed that the genotype was not significantly different from the expected distribution according to Hardy–Weinberg equilibrium in the studied patients. A chi-square test was used to compare the distribution of the TNF-α gene polymorphisms between the two groups. Odds ratios and 95% confidence intervals were calculated using unconditional logistic regression analysis that was adjusted by age, gender, duration of HD, and transfusion history. The p-value reported was two-sided and values of p < 0.05 were considered statistically significant.
RESULTS
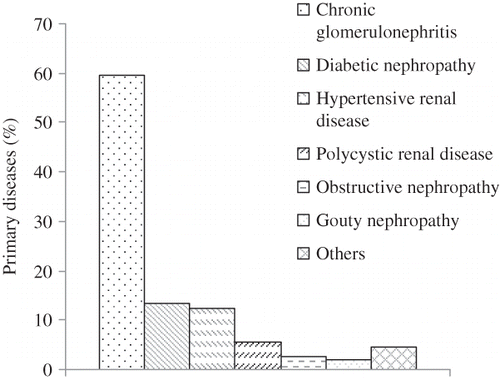
The Primary Diseases
As shown in , the main cause for end-stage renal disease in this study is chronic glomerulonephritis (59.6%), followed by diabetic nephropathy (13.5%) and hypertensive renal disease (12.6%).
Table 1. Demographic features of the anti-HCV (+) and the anti-HCV (−) groups.
Table 2. Demographic and clinical features of the persistent, viral clearance, and the anti-HCV (−) groups.
Demographic and Clinical Features
As summarized in , in the total 884 patients, 176 (19.9%) were anti-HCV (+), while 708 patients (80.1%) were anti-HCV (−). The anti-HCV (+) and anti-HCV (−) patients showed similar demographic characteristics with regard to age and gender (p > 0.05). However, the anti-HCV (+) group showed longer duration of HD (6.98 ± 4.79 years vs. 2.68 ± 2.94 years, p < 0.001), higher rate of blood transfusion (63.7% vs. 44.1%, p < 0.001), kidney transplantation (10.1% vs. 3.5%, p < 0.05), and dialyzer reuse (46.0% vs. 7.3%, p < 0.001), compared with the anti-HCV (−) group. Within the 176 anti-HCV (+) patients, 142 (80.7%) became persistent infection group. Age and gender did not differ between the persistent infection and the viral clearance groups (p > 0.05).
Interestingly, in the persistent infection group, the mean levels of serum ALT, AST, and GGT were within normal range, and only 30 (21.0%), 16 (11.5%), and 46 (33.6%) patients were with abnormal levels of serum ALT, AST, and GGT, respectively (). However, compared with the viral clearance group and the anti-HCV (−) group, the mean levels of ALT, AST, and GGT were significantly higher and the numbers of patients with abnormal levels of ALT, AST, and GGT were also markedly increased in the persistent infection group (p < 0.001).
Comparison of the Frequencies of Genotypes and Alleles
As summarized in , no significant differences in the frequencies of the two SNPs of TNF-α promoter gene could be demonstrated between the anti-HCV (+) and the anti-HCV (−) groups (p > 0.05).
Furthermore, there was no significant difference in the genotype and allele frequencies of the two SNPs of TNF-α promoter gene between the persistent infection patients and the viral clearance subjects (, p > 0.05).
DISCUSSION
Despite strict infection control policies, high prevalence of HCV infection was still reported in HD centers. It was reported that the risk factors for the high HCV infection in HD units were the duration of HD, blood transfusion, kidney transplantation, and dialyzer reuse.Citation23,24 In this study, there were 19.9% patients infected with HCV, and the high incidence was also associated with the above risk factors. However, the determiners of HCV infection may depend not only on external factors but also on the host’s condition.
Previous studiesCitation25 showed that the susceptibility to HCV infection and development of chronicity correlated with the strength and extent of T-cell response and the frequency of TNF-α and IFN-γ producing Th1-positive cell. TNF-α, one of the most important Th1 cytokines, with biological properties including immune response to infectious agents, and direct antiviral effects, may inhibit HCV replication causing lower levels of serum HCV-RNACitation26 and relate to spontaneous virus clearance.Citation10
Several studies have confirmed the associations between TNF-α gene promoter variants and the serum levels of TNF-α.Citation13,14 In considering the relationships between the TNF-α promoter gene polymorphisms and HCV infection, Chen et al.Citation15 reported an association between polymorphisms in TNF-α-308 and susceptibility to chronic HCV infection on Taiwanese general HCV-infected patients, as the A allele was more commonly found in chronic HCV patients than in healthy controls. Hohler et al.Citation27 showed that the frequency of the TNF-238-A promoter allele was significantly higher in the hepatitis C group compared to the controls.
The incidence of HCV infection is high in HD patients, and the HD patients have some differences compared with the general population. For example, the serum levels of aminotransferase in HD patients with HCV persistent infection was significantly lower than those in general patients with HCV persistent infection. This phenomenon was seen both in our study and several previous reports,Citation17,28,29 which indicated that the serum aminotransferase cut-off values should probably be modified for screening HCV infection in HD patients.
More importantly, uremic patients have impaired cell-mediated immunity and phagocytic activity, which accounts for their susceptibility to infection, malignancies, and vaccination resistance. This condition may disrupt the cytokine network by depressing Th1 response, enabling Th2-type mediators to prevail. HD patients have so unique immunity characteristics when compared with general population, and it is valuable to investigate whether the associations among the cytokine gene and hepatitis C in HD patients also exist. It may have implications in the optimal prevention of HCV in HD patients to investigate the host’s gene condition. Unfortunately, no surveys of HD patients are available yet. In this study, the possible influence of TNF-α gene promoter polymorphisms (−238 and −308) on HCV infection in 884 Chinese HD patients from 14 HD centers in south China was investigated. The result showed that the genotype distributions of TNF-α gene (−238 and −308) in the Chinese HD patients were similar to those in the general Chinese population,Citation15 but that the frequencies of A alleles and AA genotypes of TNF-α gene (−238 and −308) were lower than those in Pakistan and Ireland population.Citation30,31 Furthermore, in this study, no link was identified between the two SNPs of TNF-α promoter gene and susceptibility to HCV infection or the viral persistent infection in Chinese HD patients.
Table 3. Comparison of frequencies of genotypes and alleles of TNF-α between the anti-HCV (+) and the anti-HCV (−) groups.
Table 4. Comparison of frequencies of genotypes and alleles of TNF-α between the chronic HCV infection and the clearance of HCV infection.
The discrepancy above could be contributed to the special HD population in our study, who were different from the general population, since HD patients infected with HCV will be under the triple influence of uremia, the HD procedure, and the HCV infection itself. The chronic kidney disease could induce increased spontaneous TNF-α and IL-12 overproductions that imply an upregulation of the inflammatory activity, which were most likely due to uremia. However, HD patients showed diminished cytokines production and some, albeit not all, of their cytokine responses to a secondary stimulus Lipopolysaccharide (LPS) were consistently inhibited compared with non-HD/chronic kidney disease patients. The HCV-positive patients undergoing HD had a blunted TNF-α response compared with chronic hepatitis C patients without renal disease.Citation32,33 So with this complex condition, the protective effect may be the result of a combination of many cytokine genes, rather than the TNF-α gene only. It is possible that these two SNPs are in linkage disequilibrium with other genes or unidentified TNF-α SNPs that are actually responsible for this association. The isolated assessment of TNF-α gene polymorphisms may be misleading without considering other interacting cytokine genes. Furthermore, it is possible that different genes or combination may affect certain part of the natural history. Further investigations of multiple cytokine gene polymorphisms, such as IL-10 and IL-12, acting alone or interacting with TNF-α gene are required in the overall pathogenesis and should help define the extended haplotypes and further refine our understanding of the complex relationships. It is very likely that a complete picture of the puzzle will take a long time to complete.
Some cytokine gene polymorphisms may play important roles in determining the outcomes of HCV infection, but this only accounts for a part of the factors among the complicated immune mechanisms. Thus, other factors, such as environmental factors, need to be considered to understand the differences in the host to virus infection. So it was no surprise that no single polymorphism was more frequent in patients with HCV infection in this study. Additional studies with larger samples from various ethnic cohorts are needed to confirm the roles of cytokine gene polymorphisms in the progression of HCV infection and help the development of better protective strategies.
Declaration of Interest: This work was supported by Children’s Miracle Network Awards 2005-10, project supported by the Scientific Research Foundation of the State Human Resource Ministry for Selected Returned Overseas Chinese Scholars 2008-148, project supported by the key lab of Jiangsu Province (MMB09KF04), project supported by the Scientific Research Program of the Health Department of Jiangsu Province (H200902 and XK16200903), project supported by the National Natural Science Foundation of China (H0511-81070588), project supported by the international cooperation projects of Jiangsu Province (BZ2010058), and project supported by the funding of Blue Project 2010.
REFERENCES
- Fabrizi F, Messa P, Martin P. Impact of hemodialysis therapy on hepatitis C virus infection: A deeper insight. Int J Artif Organs. 2009;32:1–11.
- Finelli L, Miller JT, Tokars JI, Alter MJ, Arduino MJ. National surveillance of dialysis-associated diseases in the United States, 2002. Semin Dial. 2005;18:52–61.
- Sun J, Yu R, Zhu B, Wu J, Larsen S, Zhao W. Hepatitis C infection and related factors in hemodialysis patients in China: Systematic review and meta-analysis. Ren Fail. 2009;31:610–620.
- Soto-Salgado M, Perez CM, Burgos-Calderon R, Torres EA, Suarez E. Factors associated to the prevalence of antibodies to hepatitis C virus among patients receiving hemodialysis at selected dialysis centers in Puerto Rico, 2005. P R Health Sci J. 2009;28:18–23.
- Stojanovic M, Stojanovic D, Stefanovic V. The impact of malnutrition on mortality in patients on maintenance hemodialysis in Serbia. Artif Organs. 2008;32:398–405.
- Di Napoli A, Pezzotti P, Di Lallo D, . Epidemiology of hepatitis C virus among long-term dialysis patients: A 9-year study in an Italian region. Am J Kidney Dis. 2006;48:629–637.
- Machida K, Tsukamoto H, Liu JC, . c-Jun mediates hepatitis C virus hepatocarcinogenesis through signal transducer and activator of transcription 3 and nitric oxide-dependent impairment of oxidative DNA repair. Hepatology. 2010;52:480–492.
- Levrero M. Viral hepatitis and liver cancer: The case of hepatitis C. Oncogene. 2006;25:3834–3847.
- Thomas DL, Thio CL, Martin MP, . Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature. 2009;461:798–801.
- Guidotti LG, Borrow P, Hobbs MV, . Viral cross talk: Intracellular inactivation of the hepatitis B virus during an unrelated viral infection of the liver. Proc Natl Acad Sci USA. 1996;93:4589–4594.
- McCarthy JJ, Li JH, Thompson A, . Replicated association between an IL28B gene variant and a sustained response to pegylated interferon and ribavirin. Gastroenterology. 2010;138:2307–2314.
- Kanzler S, Baumann M, Schirmacher P, . Prediction of progressive liver fibrosis in hepatitis C infection by serum and tissue levels of transforming growth factor-β. J Viral Hepat. 2001;8:430–437.
- Wilson AG, Symons JA, McDowell TL, McDevitt HO, Duff GW. Effects of a polymorphism in the human tumor necrosis factor alpha promoter on transcriptional activation. Proc Natl Acad Sci USA. 1997;94:3195–3199.
- Baseggio L, Bienvenu J, Charlot C, . High LPS-stimulated TNF-alpha mRNA levels in peripheral blood mononuclear cells from non-Hodgkin’s lymphoma patients. Exp Hematol. 2001;29:330–338.
- Chen TY, Hsieh YS, Wu TT, . Impact of serum levels and gene polymorphism of cytokines on chronic hepatitis C infection. Transl Res. 2007;150:116–121.
- Thio CL, Goedert JJ, Mosbruger T, . An analysis of tumor necrosis factor α gene polymorphisms and haplotypes with natural clearance of hepatitis C virus infection. Genes Immun. 2004;5:294–300.
- Kusumoto K, Uto H, Hayashi K, . Interleukin-10 or tumor necrosis factor-alpha polymorphisms and the natural course of hepatitis C virus infection in a hyperendemic area of Japan. Cytokine. 2006;34:24–31.
- Singhal S, Kohaar I, Bharadwaj M, Shukla DK, Das BC, Kar P. Association of tumor necrosis factor-alpha gene promoter polymorphisms with acute viral hepatitis in the Indian population. Dig Dis Sci. 2010;55:1106–1112.
- Sheu JC, Lee SH, Wang JT, Shih LN, Wang TH, Chen DS. Prevalence of anti-HCV and HCV viremia in hemodialysis patients in Taiwan. J Med Virol. 1992;37:108–112.
- Lin DY, Lin HH, Hunan CC, Liaw YF. High incidence of hepatitis C virus infection in hemodialysis patients in Taiwan. Am J Kidney Dis. 1993;21:288–291.
- Taniuchi S, Masuda M, Teraguchi M, . Polymorphism of Fc gamma RIIa may affect the efficacy of gamma-globulin therapy in Kawasaki disease. J Clin Immunol. 2005;25:309–313.
- van Belzen MJ, Mulder CJ, Zhernakova A, Pearson PL, Houwen RH, Wijmenga C. CTLA4 +49 A/G and CT60 polymorphisms in Dutch coeliac disease patients. Eur J Hum Genet. 2004;12:782–785.
- Al-Jamal M, Al-Qudah A, Al-Shishi KF, Al-Sarayreh A, Al-Quraan L. Hepatitis C virus (HCV) infection in hemodialysis patients in the south of Jordan. Saudi J Kidney Dis Transpl. 2009;20:488–492.
- Nicolardi E, Grieco A, Rapaccini GL, Pompili M. Natural history, diagnosis and treatment of chronic hepatitis B and C in hemodialysis patients. G Ital Nefrol. 2010;27(3):262–273.
- Takaki A, Wiese M, Maertens G, . Cellular immune response persist and humoral responses decrease two decades after recovery from a single-source outbreak of hepatitis C. Nat Med. 2000;6:578–582.
- Dai CY, Chuang WL, Lee LP, . Associations of tumor necrosis factor alpha promoter polymorphisms at position −308 and −238 with clinical characteristics of chronic hepatitis C. J Viral Hepat. 2006;13:770–774.
- Hohler T, Kruger A, Gerken G, . Tumor necrosis factor alpha promoter polymorphism at position −238 is associated with chronic active hepatitis C infection. J Med Virol. 1998;54:173–177.
- Souza JF, Longui CA, Miorin LA, Sens YA. Gamma-glutamyltransferase activity in chronic dialysis patients and renal transplant recipients with hepatitis C virus infection. Transplant Proc. 2008;40:1319–1323.
- Lampe E, Yoshida CF, De Oliveira RV, Lauer GM, Lewis-Ximenez LL. Molecular analysis and patterns of ALT and hepatitis C virus seroconversion in hemodialysis patients with acute hepatitis. Nephrology. 2008;13:186–192.
- Abbas Z, Moatter T, Hussainy A, Jafri W. Effect of cytokine gene polymorphism on histological activity index, viral load and response to treatment in patients with chronic hepatitis C genotype 3. World J Gastroenterol. 2005;11:6656–6661.
- Barrett S, Collins M, Kenny C, Ryan E, Keane CO, Crowe J. Polymorphisms in tumour necrosis factor-alpha, transforming growth factor-beta, interleukin-10, interleukin-6, interferon-gamma, and outcome of hepatitis C virus infection. J Med Virol. 2003;71:212–218.
- Martín J, de Sequera P, Quiroga JA, . Role of hemodialysis and hepatitis C virus infection in spontaneous and induced cytokine production of patients with chronic renal disease. Cytokine. 2000;12:1248–1252.
- Spanakis NE, Garinis GA, Alexopoulos EC, . Cytokine serum levels in patients with chronic HCV infection. J Clin Lab Anal. 2002;16:40–46.