Abstract
A 50-year-old man who underwent hemodialysis (HD) at local outpatient HD center due to end-stage renal disease (ESRD) was transferred to our hospital because of pneumonia. He had severe emaciation and past history of congestive heart failure. Presenting symptoms almost consistently involved difficulty in hearing and recurrent attacks of migraine-like headaches. He was diagnosed with dilated cardiomyopathy, showing diastolic mechanical dyssynchrony by tissue Doppler echocardiography. On the day of death, he had hematemesis and hemorrhagic shock. Autopsy revealed perforation of duodenum, and genetic analysis using mitochondrial DNA from cardiac muscle and iliopsoas muscle revealed a 3243A > G mutation in the mitochondrial tRNALeu(UUR) gene, which is related to mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes (MELAS). Multiple organ failure due to the mutation of mitochondrial DNA with gastrointestinal bleeding is not a common.
INTRODUCTION
Mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes (MELAS) is a heterogeneous clinically distinct syndrome ascribed to defects in mitochondrial function.Citation1 Most patients with MELAS harbor a heteroplasmic A-to-G transition at nucleotide 3243 (3243A > G) in the transfer RNA (tRNA) leucine (UUR) gene of the mitochondrial DNA (mtDNA), which is the most common genotype of this disease.Citation2 Affected patients typically have stroke, lactic acidosis, ragged-red fibers, and encephalopathy with seizures, and dementia.Citation3 Patients with the mutation sometimes show symptoms of hypertrophic cardiomyopathy and cardiac function is rapidly impaired at some point in the clinical course, and heart failure occurs.Citation4 Renal involvement associated with MELAS syndrome with chronic renal failure (CRF) has been described in clinical symptomatology, biopsy, and molecular genetics studies,Citation5–8 and only a few cases with MELAS syndrome and gastrointestinal bleeding have been reported.Citation9
We first report here an autopsy case of MELAS syndrome with gastrointestinal hemorrhage in end-stage renal disease (ESRD).
CASE REPORT
A 50-year-old man who underwent hemodialysis (HD) at local outpatient HD center due to ESRD was transferred to our hospital because of dyspnea and high fever. He was born at full term by normal delivery and there was no abnormality during infancy. He had been in good health until he developed hearing loss at the age of 20. At the age of 37, the patient was diagnosed with proteinuria and underwent renal biopsy. Light microscopy showed almost normal and immunofluorescence microscopy showed no deposits. The renal function went gradually downhill, so that HD was initiated at the age of 46. Four years before admission, he had a low cardiac function (ejection fraction: 43% on left ventriculography) without any significant stenosis on coronary angiography. On physical examination, he had severe emaciation (height, 163 cm; body weight, 26.5 kg) and past history of congestive heart failure. His father died of stroke. His blood pressure was 82/48 mmHg and heart rate was 100 beats/min, and body temperature was 37.5°C. He had orthopnea, a third heart sound, and Levine III grade of systolic murmurs. He did not have jugular venous distention. His upper and lower extremities showed muscle atrophy but no edema.
Figure 1. (A) Chest X-ray and (B) CT scan showing a severe, widespread infiltrate shadow of right lung.
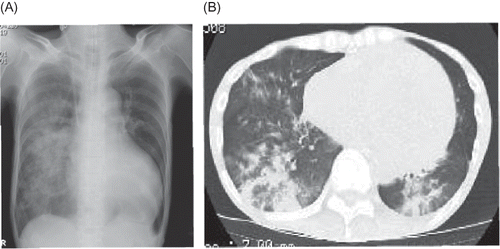
Presenting symptoms almost consistently involved difficulty in hearing and recurrent attacks of migraine-like headaches. Moist crackle was auscultated and chest X-ray and computed tomography showed infiltrate shadow in right lung (A and B). At first, we guessed the pathogenesis of these symptoms to be septic shock accompanied with ESRD and end-stage dilated cardiomyopathy. Administration of ceftriaxone (1 g), minomycin (100 mg), and imipenem/cilastatin (0.5 g) was started and continuous hemodiafiltration was initiated. Later, Pseudomonas aeruginosa and Stenotrophomonas maltophilia were detected from sputum, so that imipenem/cilastatin was switched to ciprofloxacin (200 mg) and gentamicin (120 mg).
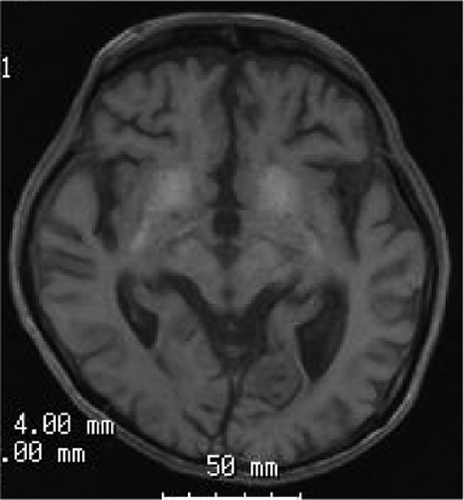
A magnetic resonance imaging scan showed diffuse cortical atrophy. The atrophy was more severe in the frontal lobes. The cerebral white matter was similarly atrophic. The calcification of the basal ganglia was noted (). A tissue Doppler echocardiography showed diastolic mechanical dyssynchrony, and so the patient was diagnosed with dilated cardiomyopathy.
Figure 2. Brain magnetic resonance imaging scans with T1 weighed images. Diffuse cortical atrophy and calcification of the basal ganglia were detected.
Laboratory examinations showed renal dysfunction. Serum levels of brain natriuretic peptide were extremely elevated. Serum lactic acid was elevated (18.8 mg/dL), but pyruvate was within normal limits. Other peripheral blood hematology and blood chemistry tests were within normal limits. Arterial blood gas analysis showed mixed acidosis ().
Table 1. Laboratory findings on admission.
The story of the present illness, past history, family history, physical and laboratory examinations, and tissue Doppler echocardiography and magnetic resonance imaging scan made us suspect mitochondrial disease. On the fourth day, intubation and artificial ventilation were conducted to improve acidosis and prevent forced respiration. Although the therapy was intensive, the status went gradually downhill. On the 52nd day, suddenly, hemorrhagic shock, and as a result, massive bloody stool, occurred. He died on the third day after bloody stool.
PATHOLOGICAL FINDINGS
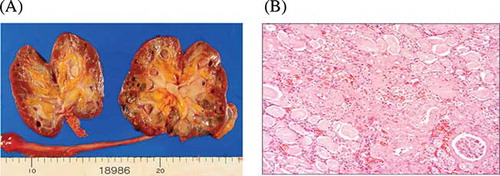
Autopsy was done 2.5 h after death. Brain edema was observed and the weight before fixation was 1224 g, but no evidence of occlusion or thrombosis of the main cerebral arteries was found. Cerebellum was atrophic (90 g). Semi-thin sections showed calcifications of the basal ganglia and thalamus. The kidneys were atrophic (right, 36 g; left, 38 g) and there were many cysts consistent with end-stage kidney. Only a few numbers of the intact glomeruli were detected by microscopic examination (A and B). The heart weighed 416 g and slight ventricular wall thickness was observed (left ventricle wall was 15 mm and right ventricle wall was 5 mm). The coronary artery was intact and patent. Microscopic examination of myocardium showed severe fibrosis. This change was prominent in epicardium side, consistent with dilated cardiomyopathy (DCM). The Congo red staining of myocardium was negative, so that amyloidosis was denied.
Figure 3. (A) Coronal section of the kidney. Autopsy revealed atrophic kidney and several renal cysts. (B) Light microscopical picture from this case. Only a few numbers of the intact glomeruli were detected. Periodic acid-Schiff stain. Original magnification ×200.
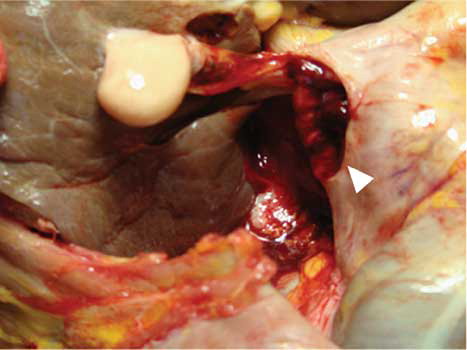
A 1 × 2 cm perforation was noticed in duodenum and confirmed the clinical suspicion of an arterio-duodenum fistula (). This perforation caused death directly. Ragged-red fibers, which are characteristic observations in mitochondrial diseases including MELAS, were not convinced in a sample from cardiac muscle and iliopsoas muscle because there were artifacts in our samples.
Genetic analysis was conducted using mtDNA from these muscles. The 3243A > G mutation was identified from both samples and mitochondrial disease was diagnosed.
DISCUSSION
Our patient presented with the symptoms of MELAS syndrome with intestinal bleeding in ESRD. The result of mtDNA analysis provided a diagnosis of MELAS. Gastrointestinal hemorrhage with MELAS syndrome, although infrequent, has been reported; however, our case is the first report of MELAS syndrome with ESRD showing gastrointestinal bleeding.
It is not common but a few reports have shown MELAS patients with kidney disease. Glomerulopathy including the nephritic-range proteinuria syndrome was noted, while biopsy-proven focal segmental glomerulosclerosis is found in some cases.Citation5–7 However, only a few cases with CRF have been reported.Citation10,11 At autopsy, our patient showed glomerulosclerosis and interstitial fibrosis consistent with ESRD, though there was no direct evidence of mitochondrial abnormality such as enlarged mitochondria with complex cristae in kidney; however, we diagnosed this case as mitochondrial kidney disease diagnosed from the symptoms of multiple organ dysfunction syndrome. Moreover, only a few reports confirmed the presence of abnormal mitochondria in the glomeruli.Citation8
Our patient died suddenly of gastrointestinal hemorrhage due to duodenum perforation and arterio-duodenum fistula. Gastrointestinal bleeding is rarely observed in MELAS. One case of gastrointestinal hemorrhage was reported in a patient with cytochrome c oxidase deficiency with MELAS.Citation9 MELAS patients sometimes suffer intracerebral hemorrhage, and fibrinoid necrosis in the small arteries was observed in hemorrhage area.Citation12 Other groups have shown that 3243 mtDNA mutation induced vascular damage, causing pancreatitis.Citation3,13 Increased production of oxygen free radicals, seen in patients with respiratory chain disorders, could inhibit the release of endothelium-derived relaxing factors that are generated by vascular endothelium leading to vasoconstriction.Citation14 Mechanism of gastrointestinal hemorrhage in MELAS is still unclear, but these abnormal changes in arteries could induce arterio-duodenum fistula.
In summary, we report a case of MELAS syndrome with gastrointestinal bleeding in ESRD. Furthermore, it is recognized that the patient has a 3243A > G mutation in the mitochondrial tRNALeu(UUR) gene.
ACKNOWLEDGMENT
We thank Kazuhiro Nagao, Toshihiko Maeda, Yoshihiro Ko, and Toshihiro Tamura for consultation on the work-up and treatment of the patient.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- DiMauro S, Moraes CT. Mitochondrial encephalomyopathies. Arch Neurol. 1993;50:1197–1208.
- Goto Y, Nonaka I, Horai S. A mutation in the tRNA(Leu)(UUR) gene associated with the MELAS subgroup of mitochondrial encephalomyopathies. Nature. 1990;348: 651–653.
- Petty RK, Harding AE, Morgan-Hughes JA. The clinical features of mitochondrial myopathy. Brain. 1986;109(5):915–938.
- Tsujita Y, Kunitomo T, Fujii M, . A surviving case of mitochondrial cardiomyopathy diagnosed from the symptoms of multiple organ dysfunction syndrome. Int J Cardiol. 2008;128:e43–e45.
- Mochizuki H, Joh K, Kawame H, . Mitochondrial encephalomyopathies preceded by de-Toni-Debre-Fanconi syndrome or focal segmental glomerulosclerosis. Clin Nephrol. 1996;46:347–352.
- Ban S, Mori N, Saito K, Mizukami K, Suzuki T, Shiraishi H. An autopsy case of mitochondrial encephalomyopathy (MELAS) with special reference to extra-neuromuscular abnormalities. Acta Pathol Jpn. 1992;42:818–825.
- Yoneda M, Tanaka M, Nishikimi M, . Pleiotropic molecular defects in energy-transducing complexes in mitochondrial encephalomyopathy (MELAS). J Neurol Sci. 1989;92:143–158.
- Yanagihara C, Oyama A, Tanaka M, Nakaji K, Nishimura Y. An autopsy case of mitochondrial encephalomyopathy with lactic acidosis and stroke-like episodes syndrome with chronic renal failure. Intern Med. 2001;40:662–665.
- Barak Y, Arnon S, Wolach B, . MELAS syndrome: Peripheral neuropathy and cytochrome C-oxidase deficiency: A case report and review of the literature. Isr J Med Sci. 1995;31:224–229.
- Matsushita T, Sano T, Nakano S, Matsuda H, Okada S. Successful mitral valve replacement for MELAS. Pediatr Neurol. 1993;9:391–393.
- Suzuki S, Hinokio Y, Hirai S, . Pancreatic beta-cell secretory defect associated with mitochondrial point mutation of the tRNA(LEU(UUR)) gene: A study in seven families with mitochondrial encephalomyopathy, lactic acidosis and stroke-like episodes (MELAS). Diabetologia. 1994;37:818–825.
- Kato H, Uchigata M, Iijima M, Shimizu S, Nonaka I, Goto Y. Fatal cerebral hemorrhage in mitochondrial encephalomyopathy. Clinical and pathological data of a case. J Neurol. 2006;253:529–530.
- Kishnani PS, Van Hove JL, Shoffner JS, Kaufman A, Bossen EH, Kahler SG. Acute pancreatitis in an infant with lactic acidosis and a mutation at nucleotide 3243 in the mitochondrial DNA tRNALeu(UUR) gene. Eur J Pediatr. 1996;155:898–903.
- Rubanyi GM, Vanhoutte PM. Superoxide anions and hyperoxia inactivate endothelium-derived relaxing factor. Am J Physiol. 1986;250:H822–H827.