Abstract
Background: Following the introduction of modified cellulosic and then synthetic membrane dialyzers, it was realized that the dialyzer bio-incompatibility depends on the membrane composition. We designed a prospective, randomized, cohort study of 6 months to determine several parameters of biocompatibility in maintenance hemodialysis (MHD) patients treated with four different membrane dialyzers. Methods: There were 60 MHD patients enrolled in the study. In baseline, synthetic low-flux dialyzer, polysulfone (PS) membrane was used in all patients for at least 3 months. Then the patients were randomly divided into three groups according to different dialyzer membranes. Synthetic high-flux dialyzer group, ployethersulfone membrane, cellulose triacetate (CTA) high-flux membrane, and synthetic low-flux dialyzer, polymethylmethacrylate (PMMA) membrane were used in 6 months. A new dialyzer was used for each study treatment, and there was no dialyzer reuse. The biocompatibility markers and solutes removal markers were detected repeatedly at different time points. Results: The blood levels of highly sensitive C reactive protein, interleukin (IL)-1β, and interleukin (IL)-13 showed no difference among different groups at al time points. However, the blood complement levels and white blood cell counts were significantly different among three groups. When the dialyzers changed from PS to PMMA membrane, C3a levels and white blood cell counts changed significantly (p < 0.05). Moreover, the changes of C5a levels were significantly different between group CTA and group PMMA in month 3 (p < 0.05). Conclusion: There were much more differences on bio-incompatibility among different dialyzer membranes.
INTRODUCTION
Following the introduction of modified cellulosic and then synthetic membrane dialyzers, it was realized that the dialyzer bio-incompatibility depended on the membrane composition. This is based on the view that relatively bio-incompatible membranes (e.g., cellulose) as compared with more biocompatible membranes (e.g., polysulfone, PS) have a higher ability to activate complement and leucocytes which may increase the inflammatory activity.Citation1 The biocompatibility of these dialyzers is evaluated by their generation of the complement activation products C3a and C5a; production of proinflammatory cytokines such as interleukin (IL)-1β, tumor necrosis factor-α (TNF-α), or IL-6; and changes in peripheral blood mononuclear cells during hemodialysis (HD).Citation2 The high morbidity and mortality of maintenance hemodialysis (MHD) patients commonly were regarded as a result of dialysis membranes bio-incapability. However, in a recent Cochrane review on this subject,Citation3 no evidence of benefit was found when biocompatible membranes were compared with cellulose/modified cellulose membranes in terms of reduced mortality or reduction in dialysis-related adverse symptoms. So it became apparent that not all adverse reactions during HD were solely due to dialyzer membrane composition, and that dialyzer design, membrane flux, anticoagulation with unfractionated heparins, dialysate water impurities, composition, and sterilants, such as ethylene oxide, could all cause sudden adverse effects during dialysis.Citation4 Furthermore, acquired immunity disturbances in HD patients are general. They are caused by uremia per se, the HD procedure, chronic renal failure complications, and therapeutic interventions for their treatment.Citation5
Table 1. Dialyzer characteristics.
In view of these considerations, we designed a prospective, randomized, cohort study to determine several parameters of biocompatibility in MHD patients treated with four different membrane dialyzers. The bio-incompatibility of them was compared during 6 months.
MATERIALS AND METHODS
Study Design
In baseline, synthetic low-flux dialyzer PS membrane (F7, Fresenius Medical Care, Bad Homburg, Germany) was used in all patients for at least 3 months. Then the patients were randomly divided into three groups according to different dialyzer membranes. Synthetic high-flux dialyzer, ployethersulfone (PES) membrane (PES-130DS, NISSHO Corporation, Osaka, Japan), cellulose triacetate (CTA) high-flux membrane (FB-130U, NISSHO Corporation), and synthetic low-flux dialyzer, polymethylmethacrylate (PMMA) membrane (B3-1.6A, Toray Industries, Inc., Tokyo, Japan) were used in 6 months. A new dialyzer was used for each study treatment, and there was no dialyzer reuse. Details of the dialyzers are presented in .
Blood samples were collected from the afferent (arterial) line. Before the first dialysis session with each of the different dialyzers mentioned (baseline), blood samples were drawn at five time points in one session, including before HD, 15, 30, 60, 240 min during HD. After the patients were transferred to be treated by three different dialyzers, respectively, blood samples were drawn at the end of 3 and 6 months. There were same five time points for collecting blood samples as above.
Patients
There were 60 MHD patients enrolled in the study in one HD unit. For inclusion in the study, patients were required to be older than 18 and less than 65 years of age and have a stable HD prescription for more than 6 months. They must have been dialyzing through a native arteriovenous fistula (AVF) providing a blood flow of 300 mL/min. Exclusion criteria included non-compliance with their dialysis prescription, using glucocorticosteroid or immunodepressant, a hemoglobin <100 g/L or active infection. The treatments of hypertension and anemia were recorded. The study was approved by the Ethics Committee of the Beijing Friendship Hospital affiliated of Capital Medical University.
Dialysis Procedure and Materials
All patients were dialyzed three times per week, 4 h per session, according to their routine HD scheme. Treatments were performed using model DBB26 dialysis delivery systems (NIKKISO, Inc., Japan). Concentration support system of dialysate was used in our unit. The fresh bicarbonate dialysate flow was 500 mL/min during HD. The endotoxin levels of fresh dialysate from dialysis machines were 0.01–0.05 EU/mL, which was detected randomly every month by limulus amebocyte lysate method. According to the individual needs of the patients, blood flow and ultrafiltration rates were kept constant 250–300 mL/min and 500–1100 mL/h, respectively. All dialyzers were prerinsed with 1000 mL 0.9% NaCl before every session. Anticoagulation was achieved with unfractionated heparin administered as an initial loading dose followed by a constant infusion. The doses of heparin were unchanged during this study.
Measurements of Solute Removal
The change in solute concentration over the entire treatment was determined from pre- and post-dialysis blood samples. Pre-dialysis blood samples were drawn from the access needle immediately following needle insertion. Post-dialysis blood samples were drawn from the arterial blood line 20 s after reducing the blood flow to 50 mL/min to mitigate any access recirculation. Concentrations of urea, creatinine (Cr), uric acid (UA), phosphorus, calcium, total cholesterol, triglyceride, highly sensitive C reactive protein (hs-CRP), and albumin were determined by standard clinical laboratory methods. The concentrations of β2-microglobulin and intact parathyroid hormone were measured by immunoradiometric assay (Immulite, Los Angeles, CA, USA). Asymmetric dimethylarginine (ADMA) levels were measured by enzyme-linked immunosorbent assay (ELISA, Adlitteram Diagnostic Laboratories, Inc., USA). Among these parameters, urea, Cr, and UA were regarded as small-molecule solutes, β2-microglobulin as a middle molecule solute, and ADMA as a protein-bonded solute.Citation6,7
The pre- to post-dialysis change in solute concentration was calculated from
Table 2. Patients’ characteristics on month 0.
Table 3. Reduction ratios (%) of several solutes among different groups.
where Cpre and Cpost are the pre- and post-dialysis solute concentrations, respectively. The post-dialysis concentrations of ADMA and β2-microglobulin were corrected for body weight (BW) according to Bergström and WehleCitation8 before calculating the pre- to post-dialysis change in concentration:
where Cpostc and Cpost are corrected and uncorrected post-HD blood concentrations of solutions, respectively, BWpost is body weight post-HD, and ΔBW is the change in body weight during dialysis.
Plasma and dialysate samples were stored at −80°C until analysis.
Assessment of Dialysis Membrane Biocompatibility
Laboratory tests of dialyzer membrane biocompatibility included plasma level of complement factors of C3a, C5a, interleukin (IL)-1β, hs-CRP, blood cells counts, and anti-inflammation factor IL-13, and so on. Blood levels of complements and interleukin factors were measured by ELISA (Adlitteram Diagnostic Laboratories). Complement levels were detected at 0, 15, 240 min; blood cell counts were detected at 0, 15, 30, 60, 240 min; and other parameters were detected at 0 and 240 min. The post-dialysis concentrations of CRP, IL-1β, and IL-13 were corrected for BW.Citation8
Statistical Analysis
Differences in plasma solute concentrations among dialyzers were assessed by analysis of variance (SPSS for Windows, version 13, SPSS, Chicago, IL, USA). Where significant differences were found (p < 0.05), differences between individual pairs of dialyzers (PES, CTA, and PMMA) were determined using the Student–Newman–Keuls correction for multiple comparisons. Paired-sample t-test method was used to compare differences of parameters between baseline and transferred dialyzers (between PS and PES, CTA, PMMA, respectively). Blood cell counts and complement factors at different time points were analyzed by repeated measures of Bonferroni method. Data were presented as mean ± SD.
RESULTS
Subject Characteristics
shows the subject characteristics among different groups. Mean age of study patients was 53.3 ± 11.7 years, 50% were men, and the average time on dialysis therapy was 8.53 ± 3.29 years. Mean systolic blood pressure was 123.5 ± 20.1 mmHg, and mean diastolic blood pressure was 72.0 ± 15.2 mmHg before dialysis in midweek. Dialysis parameters included a mean dry body weight of 64.1 ± 11.9 kg, blood flow of 300 mL/min, mean ultrafiltration volume of 3.64 ± 0.91 L in midweek session, and mean urea reduction ratio of 72.1 ± 6.9% per dialysis session. Among selected laboratory variables, mean values were 112.0 ± 16.7 g/L for hemoglobin, 895.4 ± 192.8 μmol/L for plasma Cr, 23.4 ± 4.9 mmol/L for urea, 388.7 ± 65.1 mmol/L for UA, 38.5 ± 2.2 g/L for albumin, 4.87 ± 1.17 mmol/L fortotal cholesterol, 2.43 ± 1.75 mmol/L for triglyceride, 1.96 ± 0.47 mmol/L for phosphate, 2.30 ± 0.22 mmol/L for calcium, 327.1 ± 351.2 pg/mL for intact parathyroid hormone, and 0.65 ± 0.45 mg/L for hs-CRP. There were no significant differences on above parameters among three groups ().
Solute Removal
shows the reduction ratios of different solutes according to different dialyzer groups. There were no statistically significant differences of urea, Cr, UA, and ADMA among different groups. The reduction ratio of β2-microglobulin decreased significantly when PS membrane dialyzers were changed to PES membrane dialyzers (). There were no significant differences either among three groups at baseline or transfer dialyzers from PS membrane to CTA and PMMA membranes.
Dialyzers Membrane Biocompatibility
Inflammation factors
The blood levels of hs-CRP, IL-1β, and IL-13 showed no difference among different groups at all time points ().
Table 4. Blood levels of inflammation factors.
Complements
The pattern on changes of C3a and C5a in three groups was similar. Blood levels of both C3a and C5a increased significantly at 15 min and decreased gradually at 240 min in most sessions (). However, when the dialyzer was changed from PS to PMMA membrane, the range of C3a levels were declined significantly (p < 0.05). The difference of C5a levels was not significant among 0, 15, and 240 min in month 0, when all patients dialysis were done by PS membrane dialyzer (p > 0.05). The C5a levels raised more rapidly and at higher values significantly at 15 min in group CTA than in group PMMA in month 3 (p = 0.023) ().
Table 5. Blood levels of complements.
Blood cell counts
The parameter blood cell counts first decreased at 15 min, and then increased gradually during residual period in one dialysis session. There were no significant differences in red blood cell count and platelet count, neither among groups nor among the months 0, 3, and 6 (p > 0.05) (). However, there were significant differences in white blood cell count (WBC) when dialyzer was changed from PS to PMMA membrane (p < 0.05) (). It shows that WBC in both months 3 and 6 decreased to a much lower value at 15 min and increased soon after to a much higher value and more rapidly than WBC in month 0 ().
Figure 1. Changes of blood levels on complement (C3a) (A); on complement (C5a) (B). The maintenance hemodialysis patients all were treated by polysulfone membrane dialyzers on month 0. They were divided into three different groups randomly equally according to different membranes as group PES, group CTA, and group PMMA. PES, ployethersulfone; CTA, cellulose triacetate; PMMA, polymethylmethacrylate. The number of patients in each group was 20. The patients were followed-up for 6 months. The blood levels of complement 3a (C3a) and 5a (C5a) were detected on months 0, 3, and 6. They were detected at 0, 15, and 240 min in one session on each month. The details of data are listed in Table 5.
Notes: Blood levels of both C3a and C5a increased significantly at 15 min and decreased gradually at 240 min in most sessions, except for C5a levels on month 0. The pattern on changes of C3a and C5a in three groups was similar.
*Denotes However, the range of C3a levels in group PMMA were declined significantly when the dialyzers changed from PS to PMMA membrane, both on month 3 and on month 6 (p < 0.05).
**Denotes The changes of C5a levels were significantly different between group CTA and PMMA on month 3 (p < 0.05).
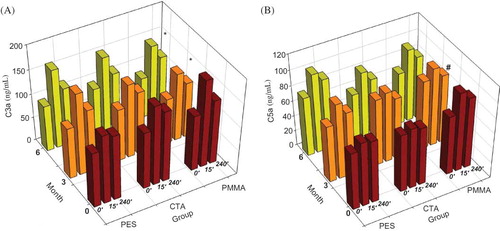
Table 6. Blood cells’ detection.
DISCUSSION
Twenty years ago, the early cuprophane dialyzers were recognized to cause pulmonary leuko sequestration associated with complement activation. This led to the term “dialyzer bio-incompatibility,” and spawned a multitude of both in vitro and in vivo studies to investigate the bio-incompatibility of different dialyzer membranes. It was commonly recognized that the biocompatibility improved from cuprophane membrane, to modified cellulose membranes, to synthetic membranes.Citation9 Today, the cost of dialyzers is very similar, whether they are of high or low flux, modified cellulose or synthetic; therefore, biocompatibility is not such a contentious issue. In our study, there were no significant differences both on most bio-incompatibility markers, such as hs-CRP, IL-1β, and platelet counts, and on anti-inflammation factor IL-13 among PS, PES, CTA, and PMMA membranes.
However, the blood complement levels and WBC counts were significantly different among three groups, the two classical parameters to characterize biocompatibility in dialysis.Citation10–12 With all three dialyzers (PES, CTA, PMMA) tested in present study, a significant increase in complement C3a and C5a levels and a minor minimal transient leucopenia within the first 15 min of dialysis was observed. There was no statistically significant difference on the changes of C5a levels during one dialysis session by using PS membrane, although the difference was significant on C3a levels. Because of the powerful adsorption capability, both relatively smaller changes of C3a levels and pronounced changes of WBC counts on PMMA group were observed in present study. These results suggested there were some differences on the biocompatibility of four dialyzers ().
Figure 2. Blood cell detection of (A) white blood cell count; (B) red blood cell count; (C) hemoglobin; (D) hematocrit; (E) platelet count.The maintenance hemodialysis patients all were treated by polysulfone membrane dialyzers on month 0. They were divided into three different groups randomly according to different membranes as group PES, group CTA, and group PMMA. PES, ployethersulfone; CTA, cellulose triacetate; PMMA, polymethylmethacrylate. The number of patients in each group was 20. The patients were followed-up for 6 months. White blood cell (WBC) count, red blood cell (RBC) count, hemoglobin (Hb), hematocrit (Hct), platelet counts (PLT) in peripheral circulation were detected on months 0, 3, and 6. The above parameters were detected at 0, 15, 30, 60, and 240 min in one session on each month. The WBC decreased significantly at 15 min and increased gradually at 30, 60, 240 min in each session. The pattern on changes of WBC in three groups was similar. Notes: *However, the WBC declined much significantly at 15 min and raised up much more quickly at 30, 60, 240 min when the patients were treated by using PMMA membrane than other membranes (p < 0.05). The other parameters also first decreased at 15 min, and then increased gradually during residual period in one dialysis session. There were no statistical differences in RBC, Hb, Hct, and PLT neither among each group nor among the months 0, 3, and 6 (p > 0.05).
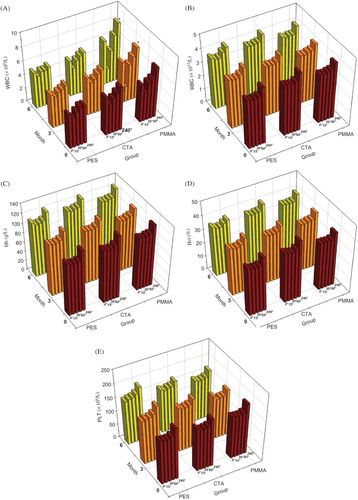
Table 7. Comparison of solute removal markers and biocompatible markers among four groups.
Three recent multicentered studies have suggested that high-flux dialyzers may convey a survival advantage. One German study looked at outcome in a group of type 2 diabetic patients, which progressed to end-stage kidney failure, and observed a stepwise increase in a cardiac composite end-point (comprising stroke, nonfatal myocardial infarction, and death from cardiac causes), both at 1 and 3 years of follow-up, with the risk steadily increasing from high-flux synthetic dialyzers to low-flux synthetic/semisynthetic dialyzers, with the highest risk associated with the use of low-flux cellulosic dialyzers.Citation13 In another study, the HEMO study was re-analyzed according to dialyzer membrane flux,Citation14 and this showed that there was a significant survival advantage for those patients randomized to high-flux dialyzers, who had been established on regular HD for more than 3.7 years, and also for those without established cerebrovascular disease. This survival advantage could either be due to improved removal of the so-called middle molecules, with β2-microglobulin acting as a surrogate for other so-called azotemic toxins, or due to improved bio-incompatibility of the membrane. The use of high-flux membranes conferred a significant survival benefit among diabetic patients and patients with serum albumin ≤4 g/dL.Citation15 Whether this improvement in patient outcomes is due to the dialyzer flux per se,Citation13,14 or improved bio-incompatibility, or to better dialysate water quality,Citation16 or center treatment effects, remains to be determined.
In our study, there were no significant differences on the clearances of small-molecule uremic toxins (Cr, urea, UA) and protein-bonded small-molecule toxin ADMA () among four dialyzer membranes. The findings of recent studies, showing no differences in ADMA elimination neither between hemodiafiltration and low-flux HDCitation17,18 nor among different membrane dialyzers,Citation18 suggest that plasma proteins that bind ADMA are larger than 40 kDa.Citation7 Whether ADMA levels reduced in the long-term treatment or modalities with increased convective transport remain to be established.
The difference of β2-microglobulin clearance was significant, which was not associated with the flux of dialyzer membranes (). The removal of β2-microglobulin was not increased, when low-flux synthetic membranes (PS membrane) transferred to high-flux modified cellulose membranes (CTA membrane). Interestingly, the removal of β2-microglobulin was even decreased, when PS dialyzer transferred to high-flux synthetic membrane (PES membrane). It might be part of reason that patients with higher β2-microglobulin level were divided into PES group (). However, there were no significant differences on the removal of β2-microglobulin between high-flux membranes (PES and CTA membranes) and low-flux synthetic membrane (PMMA membrane) in month 3 and month 6. The middle molecules such as β2-microglobulin have two clearance methods of adsorption and convection through the dialyzer membranes. It may be more important for β2-microglobulin clearance by adsorption than convection in these dialyzer membranes.Citation19 PMMA membranes have more powerful adsorptive ability than other membranes.Citation19–21 So the low-flux synthetic membranes with higher absorptive ability (PS/PMMA membrane) may have the similar capability to high-flux membranes (PES/CTA membranes) on β2-microglobulin removal. It is obvious that the possibilities of up-to-date polymer chemistry allow for the development of large-pore membranes with different compositions and characteristics. It is known that various high-flux membranes differ in biocompatibility toward leukocytes and complement system, coagulator properties, and/or adsorptive capacity.Citation22 Hence, although this question has rarely been addressed, it is conceivable that there are also differences in removal capacity.
Whether the biocompatibility and the removal capacity are important enough to be translated into differences in clinical conditions and outcome remains open for discussion and should be evaluated. As Cheung said, a certain bio-incompatible phenomenon can be further classified as beneficial or deleterious depending on its biological effects as well as its acute and chronic impacts on the dialysis patient.Citation11
Our study had several limitations. First, our study population was small, and the power to detect differences was, therefore, limited. Second, the follow-up time was limited, and we have no information on the clinical outcome affected by dialyzer membranes’ bio-incompatibility beyond 6 months. Third, although we used some markers of biocompatibility, there is as yet limited information on their prognostic significance. Fourth, one group using PS membrane might be set as the control group in order to decrease biases.
Declaration of interest: The authors thank Zhang Yu and Zhang Qi-Dong for their technical help. This study got financial support from Beijing Municipal Science and Technology Commission Funds (No. D09050704310903), Collaborative Project Funds on Fundamental and Clinical Research of Capital Medical University (No. 10JL26), the Research Fund for the Doctoral Program of Higher Education (PFDP) of Ministry of Education of the People’s Republic of China (20101107120003).
REFERENCES
- Bowry SK, Kuchinke-Kiehn U, Ronco C. The cardiovascular burden of the dialysis patient: The impact of dialysis technology. Contrib Nephrol. 2005;149:230–239.
- Varela MP, Kimmel PL, Phillips TM, Biocompatibility of hemodialysis membranes: Interrelations between plasma complement and cytokine levels. Blood Purif. 2001;19:370–379.
- MacLeod A, Daly C, Khan I, Comparison of cellulose, modified cellulose and synthetic membranes in the hemodialysis of patients with end-stage renal disease. Cochrane Database Syst Rev. 2001;(3):CD003234.
- Davenport A. Intradialytic complications during hemodialysis. Hemodial Int. 2006;10:162–167.
- Eleftheriadis T, Antoniadi G, Liakopoulos V, Kartsios C, Stefanidis I. Disturbances of acquired immunity in hemodialysis patients. Semin Dial. 2007;20(5):440–451.
- Boger RH, Zoccali C. ADMA: A novel risk factor that explains excess cardiovascular event rate in patients with end-stage renal disease. Atheroscler Suppl. 2003;4:23–28.
- Kielstein JT, Boger RH, Bode-Boger SM, Low dialysance of asymmetric dimethylarginine (ADMA)—In vivo and in vitro evidence of significant protein binding. Clin Nephrol. 2004;62:295–300.
- Bergström J, Wehle B. No change in corrected beta 2-microglobulin concentration after cuprophane hemodialysis. Lancet. 1987;1:628–629.
- Krieter DH, Lemke HD, Wanner C. A new synthetic dialyzer with advanced permselectivity for enhanced low-molecular weight protein removal. Artif Organs. 2008;32:547–554.
- Wingard RL, Husni L, Effect of the membrane biocompatibility on nutritional parameters in chronic hemodialysis patients. Kidney Int. 1996;49:551–556..
- Cheung AK. Biocompatibility of hemodialysis membranes. J Am Soc Nephrol. 1990;1:150–161.
- Martin-Malo A, Castillo D, Castro M, Biocompatibility of dialysis membranes: A comparative study. Nephrol Dial Transplant. 1991;6(Suppl. 2):55–58.
- Krane V, Krieter DH, Olschewski M, Dialyzer membrane characteristics and outcome of patients with type 2 diabetes on maintenance hemodialysis. Am J Kidney Dis. 2007;49:267–275.
- Delmez JA, Yan G, Bailey J, Cerebrovascular disease in maintenance hemodialysis patients: Results of the HEMO Study. Am J Kidney Dis. 2006;47:131–138.
- Locatelli F, Martin-Malo A, Hannedouche T, Effect of membrane permeability on survival of hemodialysis patients. J Am Soc Nephrol. 2009;20(3):645–654.
- Schiffl H, Lang SM, Stratakis D, Effects of ultrapure dialysis fluid on nutritional status and inflammatory parameters. Nephrol Dial Transplant. 2001;16:1863–1869.
- Kalousova M, Kielstein JT, Hodkova M, No benefit of hemodiafiltration over hemodialysis in lowering elevated levels of asymmetric dimethylarginine in ESRD patients. Blood Purif. 2006;24:439–444.
- Grooteman MP, Wauters IM, Teerlink T, Plasma dimethylarginine levels in chronic hemodialysis patients are independent of the type of dialyzer applied. Blood Purif. 2007;25:281–289.
- Aoike I. Clinical significance of protein adsorbable membranes–long-term clinical effects and analysis using a proteomic technique. Nephrol Dial Transplant. 2007;22(Suppl 5):v13–v19.
- Yamashita AC, Tomisawa N. Importance of membrane materials for blood purification devices in critical care. Transfus Apher Sci. 2009;40:23–31.
- Yamashita AC, Tomisawa N. Membrane materials for blood purification in critical care. Contrib Nephrol. 2010;166:112–118.
- Opatrny K Jr, Krouzzecky A, Polanska K, Does an alteration of dialyzer design and geometry affect biocompatibility parameters. Hemodial Int. 2006;10:201–208.
