Abstract
Background: Acute kidney injury (AKI) requiring dialysis commonly occurs in critically ill patients and is associated with high mortality. Factors impacting outcomes of individuals with AKI who underwent continuous renal replacement therapy (CRRT), including early versus late initiation and duration of CRRT, were examined. Methods: Survival and recovery of renal function for patients with AKI in the intensive care unit were retrospectively examined over a 7-year period. Factors associated with mortality and renal recovery were analyzed based on severity of illness as defined by Cleveland Clinic Foundation (CCF) score. Univariate and multivariate logistic regression analysis with backward elimination was performed to determine the most significant risk factors. Results: Of patients who underwent CRRT, 230/330 met inclusion criteria. During index admission 112/230 (48.7%) patients died. Median survival was 15.5 days [95% confidence interval (12.0, 18.0)]. Among survivors, renal recovery occurred in 84/118 (71.2%). Renal recovery overall was observed in 90/230 subjects (39.13%). A higher baseline CCF score correlated with higher mortality and lower probability of renal recovery. Patients initiated on CRRT > 6 days after AKI diagnosis had significantly higher mortality compared with those initiated earlier (odds ratio = 11.66, p = 0.0305). Patients receiving CRRT >10 days had a higher mortality rate compared with those with shorter exposure (71.3% vs. 45.5%, respectively, p = 0.012). Conclusions: CRRT remains an important dialysis modality in hemodynamically unstable patients with AKI. Mortality in these patients continues to be high. Renal recovery is high in survivors. Delay in initiation and length of CRRT exposure may portend poorer prognosis.
INTRODUCTION
Acute kidney injury (AKI) is a frequent complication encountered in critically ill patients, with a reported incidence rate ranging from 6% to 67%.Citation1,2 In most patients the condition is transient with improvement in renal function following abatement of underlying abnormalities. However, a significant percentage of these patients in intensive care units (ICUs) will ultimately develop multi-organ dysfunction and require renal replacement therapy (RRT). Despite advances in medical technology and increases in the quality of patient care, the mortality rate remains high (50–70%) in patients requiring RRT.Citation3–6
Patients with AKI requiring dialysis can be treated by either intermittent hemodialysis (IHD) or continuous RRT (CRRT). While IHD is technically simpler and less labor intensive and therefore more cost effective, it is more difficult to perform on hemodynamically unstable patients due to the intensity and abrupt changes in fluid volume associated with this procedure. In these situations, CRRT is the optimal treatment modality because it is more effective in correcting acid–base and electrolyte abnormalities, clearing solutes, and removing pro-inflammatory mediators, the latter being especially important in the context of sepsis.Citation5,7–10
The choice of RRT modality for critically ill patients has remained controversial in nephrology literature through the past decade. While some studies have shown a survival benefit with use of CRRT,Citation11–13 most randomized controlled trials and meta-analyses have failed to show a survival benefit with CRRT compared with IHD.Citation14–17 Significant benefit of one modality over another in terms of outcomes of renal recovery, long-term dialysis independence, and factors impacting those outcomes remain unclear. Further study is required to provide definitive answers.
As CRRT becomes more widely available and as experience with its use increases, it is imperative to reevaluate the outcomes of patients undergoing the treatment. A retrospective study was initiated to examine outcomes of individuals with AKI who underwent CRRT. Survival and renal recovery outcomes were reviewed for ICU patients with AKI in central Wisconsin at Marshfield Clinic/St. Joseph’s Hospital (MC/SJH), a tertiary care teaching hospital. This study reviewed the historical experience over a 7-year period spanning January 1999 to February 2006 and analyzed factors that are associated with mortality and renal recovery in these patients.
A sub-aim of this study was to analyze CRRT data in critically ill patients at MC/SJH following stratification of patients by severity of illness. Stratification was achieved by applying the Cleveland Clinic Foundation (CCF) scoring system developed by Paganini et al.Citation18 at the CCF, another experienced CRRT provider, for predicting outcomes in patients with AKI.
MATERIALS AND METHODS
Environment
This study was undertaken at MC/SJH, a tertiary care teaching hospital in central Wisconsin with over 500 licensed beds and a certified level II trauma center. Patients in this study were admitted to either the medical ICU or surgical ICU between January 1999 and February 2006. CRRT has been used by MC/SJH’s Department of Nephrology since 1999.
Study Design
This study was designed as a retrospective analysis of CRRT outcomes at MC/SJH and approved by the Marshfield Clinic Research Foundation’s Institutional Review Board. As part of this evaluation, the CCF score was applied as a tool for stratifying illness severity and patient outcomes for patients within our system of care.
The inclusion criteria were developed to include patients whose available clinical data aligned with parameters captured in generating a CCF score.
Inclusion Criteria
| 1. | Diagnosis of acute renal failure (if creatinine at admission <1.4 mg/dL, AKI was diagnosed when creatinine ≥ 2.0 mg/dL; if creatinine at admission >1.5 mg/dL, AKI was diagnosed when creatinine ≥2.5 mg/dL) | ||||
| 2. | Admission to ICU | ||||
| 3. | CRRT (continuous veno-venous hemodialysis/filtration or continuous arterio-venous hemodialysis/filtration) | ||||
Exclusion Criteria
| 1. | Renal transplant | ||||
| 2. | End-stage renal disease, on dialysis | ||||
| 3. | CRRT delivered for less than 24 h | ||||
| 4. | CRRT delivered for reasons other than AKI | ||||
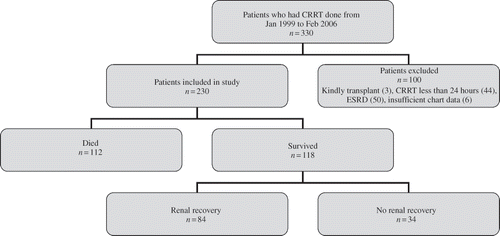
Patients (n = 330) who underwent CRRT from January 1999 to February 2006 at MC/SJH were evaluated for inclusion in this study, and 230 met the inclusion criteria. The remaining 100 patients met one or more of the following exclusion criteria: history of kidney transplant (n = 3), history of end-stage renal disease (n = 50), duration of CRRT less than 24 h (n = 44), or incomplete data in their medical record (n = 6) (). Three patients met more than one exclusion criteria.
Figure 1. Flow diagram of patients (n = 330) who underwent CRRT from January 1999 to February 2006 at the MC/SJH. Renal recovery defined as the absence of need for dialysis at time of hospital discharge.
CRRT orders were initiated by the consulting nephrologist and implemented by ICU nurses in consultation with SJH’s dialysis nursing service. CRRT (predominantly continuous veno-venous hemodiafiltration) was performed with the Prisma machine (Prisma, Gambro Americas, Lakewood, CO, USA) until 2001 and was then transitioned to the Fresenius system (Fresenius Medical Care North America, Waltham, MA, USA). No significant changes in the prescription or practice of CRRT occurred with the change in the system. The usual practice at MC/SJH included a blood flow rate of 150–200 mL/min, with the replacement fluid run in the prefilter position at a rate of approximately 25–30 mL/kg/h. Citrate was used for regional anticoagulation unless contraindicated. Changes in blood and replacement fluid flow rates and dialysate composition as well as type of anticoagulant were dictated by the patients’ clinical condition.
Data were abstracted from the ICU daily care nursing records. Laboratory data were extracted from MC/SJH combined electronic medical record. An extensive database was developed to capture the following parameters: demographics (gender, age, admission dates), CRRT data (time of onset, duration, vascular access, anticoagulation, complications), cause of AKI, comorbidities, multisystem involvement, and survival and renal recovery status for the index hospitalization. Data abstracted included all elements necessary to satisfy all components required for the CCF score calculation. Details of mean arterial pressures (MAP), total fluid balances (including urine outputs), and use and/or change in doses of vasopressor drugs were documented for 12 h before and 72 h after the start of CRRT and were used as indicators of efficacy of CRRT and hemodynamic stability of patients.
The primary endpoint was hospital mortality in treated patients. The secondary endpoints were renal recovery (defined as the absence of dialysis dependence at the time of hospital discharge) and determinants of mortality or renal recovery in these patients. Association of time lapse between diagnosis of AKI and CRRT initiation, total duration of CRRT, and primary and secondary outcomes were additionally analyzed. A CCF score was calculated for each patient at the time of CRRT initiation, and the outcomes were further analyzed following stratification of patients by severity of illness.
Statistical Analysis
Descriptive statistics were used to summarize the baseline characteristics including mean, median, standard deviation, frequency, and percentage. Univariate analyses including logistic regression analysis, chi-square test, Fisher’s exact test, and Wilcoxon rank sum test were applied to identify and select significant risk factors for the outcomes of death and renal recovery. Multivariate logistic regression analysis with backward elimination method was performed to determine the most significant risk factors.
RESULTS
As summarized in , 230/330 patients underwent CRRT at MC/SJH during the time frame between 1999 and 2006 and met the inclusion criteria. The demographics of our patient population, causes of AKI, and outcomes are summarized in . During the index admission, 112 patients (48.7%) died. The median survival time from time of admission was 15.5 days [95% confidence interval (CI) 12.0, 18.0]. Renal recovery, defined as no dialysis dependence at discharge, was documented for 84/118 (71.2%) of the surviving patients (39.13% of total subjects included in study).
Results of mortality, median survival time, and renal recovery were further analyzed by stratifying subjects on the basis of CCF score (). As anticipated, the mortality rate increased proportionally with CCF score, suggesting multi-organ involvement and worsening clinical condition. Poor outcomes showed high correlation with initial severity of disease before CRRT initiation as confirmed by high initial CCF score. Potential for renal recovery decreased as the pre-CRRT CCF score increased.
Table 1. Baseline demographics and clinical outcomes for patients with acute renal failure requiring CRRT at Marshfield Clinic between 1999 and 2006 (n = 230).
Univariate analysis () and multivariate logistic regression analysis were performed on factors impacting mortality. The most significant results are summarized in . Important factors that were identified in the analysis that significantly increased mortality included the presence of a Do Not Resuscitate (DNR) order, initiation on CRRT >6 days after diagnosis of AKI, presence of neurological failure (new cerebrovascular accident-ischemic/intracranial bleed, encephalopathy), persistence of vasopressor use by third day of CRRT, and higher creatinine on initiation of therapy. Requirement of intermittent dialysis after discontinuation of the continuous replacement therapy up to time of discharge from hospital correlated strongly with mortality during the index hospitalization.
The analysis revealed additional unique factors for MC/SJH data that were not represented in the CCF score. Outcomes in mortality and renal recovery were assessed in relation to the time elapsed between the diagnosis of AKI and the initiation of CRRT (AKI–CRRT) and the total duration of CRRT using univariate and multivariate analyses. Univariate analysis revealed that patients with AKI–CRRT time of <6 days had a significantly lower risk of mortality [odds ratio (OR)= 0.198, p = 0.0134] compared with the patients with AKI–CRRT ≥6 days. In addition, the cohort of patients with CRRT duration ≤9 days had a significantly lower risk of mortality (OR = 0.335, p = 0.0131) compared with those on CRRT for ≥10 days (). However, univariate analysis for association between CRRT duration and AKI–CRRT and renal recovery failed to reach statistical significance (). Multivariate logistic regression analysis confirmed AKI–CRRT as an independent risk factor for mortality ().
Table 2. Mortality rate, median survival time, and renal recovery rate classified by CCF score.
Table 3. Risk factors for mortality considered and analyzed using univariate analysis.
Table 4. Odds ratio, 95% CI, and p-values for six factors determined from multivariate logistic regression analysis of risk factors for mortality.
Table 5. Logistic regression analysis or nonparametric analysis for renal recovery.
Based on a detailed abstraction of hourly blood pressures, the baseline (12 h prior to onset of CRRT) MAP was 75.4 ± 11.4 mmHg, and the mean MAP over the next 72 h was 77.2 ± 10.5 mmHg. The change in the MAP from baseline was insignificant (p = 0.2142). At the time of CRRT initiation, 49% of subjects were on vasopressor agents (mean 0.8 ± 1.0 drugs) (). Median urinary outputs on the first 3 days of CRRT were 400, 127, and 88 mL. The median cumulative total fluid balance during 3 days of CRRT was −2153 mL. Neither an increase in MAP reduction nor lower baseline urinary output levels correlated significantly with renal recovery. Similarly, neither the number of vasopressors nor the doses of vasopressors was found to influence duration of CRRT, renal recovery, or mortality ().
DISCUSSION
Critically ill patients commonly experience AKI. Despite advances in technology and more widespread use of RRT for these patients, mortality for AKI patients requiring RRT continues to be in the 50%–70% range.Citation1,13,14 It is currently unclear whether IHD versus CRRT has survival benefits or impacts recovery of renal function in survivors. While some studies suggest a survival benefit with use of CRRT,Citation10,12,15 subsequent studies have reported no survival benefit attributable to use of either modality.Citation5,6,16–20 This issue has been addressed by three meta-analyses as well as by a Cochrane Library review.Citation9,14,15,21 Despite differences in study designs, the overall conclusion from the meta-analyses was that the choice of RRT modality in critically ill AKI patients did not affect mortality.
Results from our study and othersCitation5,22,23 further validate that mortality in patients with AKI, especially those requiring CRRT, is extremely high (48.7%). Renal recovery to a non–dialysis-dependant state following AKI that required RRT has been reported as high as 95%. In our study, 84/118 (71.2%) of surviving patients recovered adequate renal function and did not require long-term outpatient dialysis. Indeed, most patients who survive the initial episode of AKI will recover enough renal function to avoid long-term dialysis. While the absence of renal recovery (dialysis dependence at discharge) very significantly correlated with mortality, it is more so an outcome measure and cannot be used as a predictor of mortality early in the course of the AKI.
Various studies have shown the use of CRRT as an independent predictor of renal recovery in survivors;Citation6,19,24 however, meta-analyses have failed to support this finding. As CRRT is chosen by default as the modality for critically ill patients located in ICU, comparison of outcomes from this treatment must include assessment of the severity of the patient’s illness using a scoring system designed for that purpose. Better understanding of disease mechanisms in the last two decades has led to a better understanding of the role of abnormal physiologic parameters in quantification of disease severity and associated outcomes. While multiple scores for assessing the severity of illness have been developed (acute physiology and chronic health evaluation (APACHE) I, II, III, IV, simplified acute physiology scores (SAPS) I, II, III, sequential organ failure assessment (SOFA), mortality probability models (MPM)),Citation25–30 these were not specifically designed to predict outcomes of AKI patients, nor have they been satisfactorily validated for this purpose. Since comorbid conditions significantly impact the final outcomes of AKI patients, we tested application of the CCF scale on our index population. The CCF score, as developed by Paganini et al.,Citation18 was used to calculate illness severity and predict mortality for ICU patients with AKI requiring dialysis. The CCF scoring method was initially developed based on data from 512 patients in the Cleveland Clinic Registry database. The risk factors and variables used for score generation (range 1–20) and odds ratios for mortality are listed in . A higher score implies multi-organ involvement with worsening severity of disease. This scoring system was used in this study by Augustine et al.Citation5 in a randomized controlled trial comparing IHD to CRRT, wherein patients were stratified by severity of illness. The association between worsening clinical status and multi-organ failure correlated with increase in CCF score in patients treated with CRRT and mortality.
Table 6. Cleveland Clinic Foundation (CCF) score calculation methodology.
In this study mortality rates correlated with increasing severity in illness, as indicated by an increase in CCF score. The mortality rates approximately doubled with increasing morbidity from 39% in the CCF score 1–7 to 76% in the CCF score 13–17 range. Similarly, the median survival time decreased from 19 to 17 days in the same CCF score ranges ().
Recently, the Risk of renal dysfunction, Injury to the kidney, Failure of kidney function, Loss of kidney function, and End-stage kidney disease (RIFLE) criteria were proposed for the diagnosis of AKI by the Acute Dialysis Quality Initiative. Though proposed as a classification system for AKI, the criteria have been used variably by investigators across the world as an outcome measure in AKI.Citation31,32 The validity of the use of these criteria in patients undergoing dialysis is still debated.Citation33,34 These criteria are recent and were not operative during the time interval of our study; hence to maintain uniformity with the CCF score development criteria, the inclusion criteria (especially for diagnosis of AKI) for this study were chosen to reflect those used to develop the CCF score.
Interestingly, the time elapsed between diagnosis of AKI and initiation of CRRT showed significant bearing on mortality. When patients with AKI diagnosis to CRRT time of ≥6 days were compared with those with AKI diagnosis to CRRT time of <6 days, no significant difference in CCF score was noted (Wilcoxon rank sum test p= 0.7333). However, there was a statistically significant difference in mortality implying that early initiation of CRRT exerts a potentially significant impact on mortality (OR = 11.66, p = 0.035, ), independent of initial severity of disease, as suggested by lack of a significant difference in the CCF scores in the two subgroups. The issue of early start of CRRT has been addressed in a few studies in the past. Gettings et al.Citation35 reported improvement in survival with early onset of CRRT, though the study was limited by use of length of hospital stay before CRRT rather than date of diagnosis of AKI. The study was conducted on 100 trauma patients with blood urea nitrogen >60 mg/dL as an indication for CRRT. By contrast, Bouman et al.Citation36 did not report any survival benefit with use of early CRRT in AKI patients. In their study, oliguria with urine output <30 mL/h was used to define AKI.Citation36 There have been variable reports of survival benefit with early high-volume ultrafiltration in patients with refractory septic shock (though not necessarily with AKI). To our knowledge, our study presents the largest cohort of patients with a diagnosis of AKI who underwent CRRT in which a mortality benefit is reported with early (<6 days) initiation of CRRT. In contrast, patients requiring longer duration of CRRT (>10 days, suggesting prolonged hemodynamic instability) had higher mortality (OR = 2.99, p = 0.0131, ). Duration of CRRT did not have a statistically significant impact on renal recovery.
Multivariate analysis of risk factors for the primary endpoint of mortality identified some new factors, including absence of renal recovery, presence of a DNR order, initiation on CRRT more than 6 days, and neurological failure (). A study by Wald et al.Citation37 defined baseline non-oliguria, higher renal function, and a shorter CRRT period as predictors of improved renal outcomes. However, logistic regression analysis or nonparametric analysis for renal recovery revealed only two significant factors in our study: age and CCF_score. All other factors (baseline urine output, baseline MAP, use of pressors, or change in MAP over 3 days of CRRT) did not achieve significance ( and ).
Table 7. Odds ratio (OR), 95% confidence interval (CI), and p-values for two factors determined from multivariate logistic regression analysis of renal recovery.
CRRT remains an excellent well-tolerated modality for renal replacement in the presence of AKI in the ICU patient, especially in the hemodynamically unstable patient where IHD may not be feasible. Prospective studies in larger cohorts of patients are necessary to settle the uncertainty regarding mortality risk and likelihood of renal recovery associated with use of either modality.Citation11–17 This study contributes evidence-based data on a relatively large pool of patients studied over 7 years and may contribute to the derivation of more definitive answers. The use of the CCF score before initiation of CRRT in a patient with prolonged nonrecovery of renal function during an ICU stay may inform patient management decisions for these critically ill patients.
Limitations
Our study had some fundamental limitations due to its retrospective design. Many patients had to be excluded from this study due to lack of consistency of data recording. Patients requiring CRRT for less than 24 h were excluded from this study, which may have excluded a cohort of individuals with a critical (often fatal) condition, possibly introducing a bias toward more favorable outcomes. Furthermore, there is an inherent lead time bias connected to the retrospective design for the timing of the initiation of RRT. Though the principles of practice of CRRT are fairly standard, there can be minor variations among individual prescriptions by different nephrologists. Averaging data over a period of time should marginalize that bias. It is important to recognize that the CCF scoring system was developed in a population with demographics different from the population in our study. Such differences may contribute to observation of lower CCF scores on average at MC/SJH, where the highest CCF score noted was 17, compared with 20 as the highest CCF score at Cleveland Clinic. Further, institutional management relative to CRRT initiation may differ in some aspects across the institutions, and this may be reflected in the difference in CCF scores observed across the institutions.
CONCLUSION
In summary, retrospective analysis of MC’s data, documenting their experience with CRRT in critically ill AKI patients, demonstrates the following:
| 1. | High rate of mortality associated with AKI, especially when renal replacement is required. The predictability of higher mortality is associated with a higher illness severity score at the time of initiation of CRRT, as measured by the CCF scoring system. | ||||
| 2. | An increased risk of mortality from a delay in initiation of CRRT. | ||||
| 3. | Increased risk of mortality with longer duration of CRRT. | ||||
ACKNOWLEDGMENTS
The authors greatly appreciate the efforts of Ingrid Glurich, PhD, of the Marshfield Clinic Research Foundation’s Office of Scientific Writing and Publication in editing and critical review of the manuscript. The efforts of the Marshfield Clinic data abstraction team in performing such an extensive data abstraction are greatly appreciated. We also thank Jeanne Schreiner, RN, for providing us with all the information regarding the dialysis machines and the technical aspects of CRRT and Dr. Sevag Demirjian, Cleveland Clinic, for facilitating access to the original data for CCF score calculation and study by Augustine et al. Funding for this study was provided by the Marshfield Clinic, Physician Research Funds.
Declaration of interest: HSV, RAD, TRO, and HL are employees of Marshfield Clinic. Authors report no real or perceived conflict of interest.
REFERENCES
- Uchino S, Kellum JA, Bellomo R, Acute renal failure in critically ill patients: A multinational, multicenter study. J Am Med Assoc. 2005;294(7):813–818.
- Hoste EA, Clermont G, Kersten A, RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: A cohort analysis. Crit Care. 2006;10(3):R73.
- Barton IK, Hilton PJ, Taub NA, Acute renal failure treated by hemofiltration: Factors affecting outcome. Q J Med. 1993;86(2):81–90.
- Chertow GM, Christiansen CL, Cleary PD, Munro C, Lazarus JM. Prognostic stratification in critically ill patients with acute renal failure requiring dialysis. Arch Intern Med. 1995;155(14):1505–1511.
- Augustine JJ, Sandy D, Seifert TH, Paganini EP. A randomized controlled trial comparing intermittent with continuous dialysis in patients with AKI. Am J Kidney Dis. 2004;44(6):1000–1007.
- Mehta RL, McDonald B, Gabbai FB, A randomized clinical trial of continuous versus intermittent dialysis for acute renal failure. Kidney Int. 2001;60(3):1154–1163.
- De Vriese AS, Colardyn FA, Philippé JJ, Vanholder RC, De Sutter JH, Lameire NH. Cytokine removal during continuous hemofiltration in septic patients. J Am Soc Nephrol. 1999;10(4):846–853.
- Jakob SM, Frey FJ, Uehlinger DE. Does continuous renal replacement therapy favourably influence the outcome of the patients? Nephrol Dial Transplant. 1996;11(7):1250–1255.
- Rabindranath KS, Adams J, MacLeod AM, Muirhead N. Intermittent Versus Continuous Renal Replacement Therapy for Acute Renal Failure in Adults (Review). The Cochrane Collaboration; Issue 4. Hoboken, NJ: John Wiley & Sons, Ltd; 2008.
- Kellum JA, Angus DC, Johnson JP, Continuous versus intermittent renal replacement therapy: A meta-analysis. Intensive Care Med. 2002;28(1):29–37.
- Swartz RD, Messana JM, Orzol S, Port FK. Comparing continuous hemofiltration with hemodialysis in patients with severe acute renal failure. Am J Kidney Dis. 1999;34(3):424–432.
- Ronco C, Kellum JA, Mehta R. Acute dialysis quality initiative (ADQI). Nephrol Dial Transplant. 2001;16(8):1555–1558.
- Swartz RD, Bustami RT, Daley JM, Gillespie BW, Port FK. Estimating the impact of renal replacement therapy choice on outcome in severe acute renal failure. Clin Nephrol. 2005;63(5):335–345.
- Pannu N, Klarenbach S, Wiebe N, Manns B, Tonelli M. Alberta kidney disease network. Renal replacement therapy in patients with acute renal failure: A systematic review. J Am Med Assoc. 2008;299(7):793–805.
- Bagshaw SM, Berthiaume LR, Delaney A, Bellomo R. Continuous versus intermittent renal replacement therapy for critically ill patients with acute kidney injury: A meta-analysis. Crit Care Med. 2008;36(2):610–617.
- Uehlinger DE, Jakob SM, Ferrari P, Comparison of continuous and intermittent renal replacement therapy for acute renal failure. Nephrol Dial Transplant. 2005;20(8):1630–1637.
- Lins RL, Elseviers MM, Van der Niepen P, Intermittent versus continuous renal replacement therapy for acute kidney injury patients admitted to the intensive care unit: Results of a randomized clinical trial. Nephrol Dial Transplant. 2009;24(2):512–518.
- Paganini EP, Halstenberg WK, Goormastic M. Risk modeling in acute renal failure requiring dialysis: The introduction of a new model. Clin Nephrol. 1996;46(3):206–211.
- Uchino S, Bellomo R, Kellum JA, Patient and kidney survival by dialysis modality in critically ill patients with acute kidney injury. Int J Artif Organs. 2007;30(4):281–292.
- Vinsonneau C, Camus C, Combes A, Continuous venovenous hemodiafiltration versus intermittent hemodialysis for acute renal failure in patients with multiple-organ dysfunction syndrome: A multicentre randomised trial. Lancet. 2006;368(9533):379–385.
- Tonelli M, Manns B, Feller-Kopman D. Acute renal failure in the intensive care unit: A systematic review of the impact of dialytic modality on mortality and renal recovery. Am J Kidney Dis. 2002;40(5):875–885.
- Bell M, Liljestam E, Granath F, Fryckstedt J, Ekbom A, Martling CR. Optimal follow-up time after continuous renal replacement therapy in actual renal failure patients stratified with the RIFLE criteria. Nephrol Dial Transplant. 2005;20(2):354–360.
- Maccariello E, Soares M, Valente C, RIFLE classification in patients with acute kidney injury in need of renal replacement therapy. Intensive Care Med. 2007;33(4):597–605.
- Bell M, SWING, Granath F, Schön S, Ekbom A, Martling CR. Continuous renal replacement therapy is associated with less chronic renal failure than intermittent hemodialysis after acute renal failure. Intensive Care Med. 2007;33(5):773–780.
- Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: A severity of disease classification system. Crit Care Med. 1985;13(10):818–829.
- Knaus WA, Wagner DP, Draper EA, The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991;100(6):1619–1636.
- Zimmerman JE, Kramer AA, McNair DS, Malila FM. Acute Physiology and Chronic Health Evaluation (APACHE) IV: Hospital mortality assessment for today’s critically ill patients. Crit Care Med. 2006;34(5):1297–1310.
- Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. J Am Med Assoc. 1993;270(24):2957–2963.
- Metnitz PG, Moreno RP, Almeida E, SAPS 3—From evaluation of the patient to evaluation of the intensive care unit. Part 1: Objectives, methods and cohort description. Intensive Care Med. 2005;31(10):1336–1344.
- Moreno RP, Metnitz PG, Almeida E, SAPS 3—From evaluation of the patient to evaluation of the intensive care unit. Part 2: Development of a prognostic model for hospital mortality at ICU admission. Intensive Care Med. 2005;31(10):1345–1355.
- Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute dialysis quality initiative workgroup. Acute renal failure – Definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8(4):R204–R212.
- Mehta RL, Kellum JA, Shah SV, Acute kidney injury network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31.
- Uchino S, Bellomo R, Morimatsu H, External validation of severity scoring systems for acute renal failure using a multinational database. Crit Care Med. 2005;33(9):1961–1967.
- Ahlström A, Kuitunen A, Peltonen S, Comparison of 2 acute renal failure severity scores to general scoring systems in the critically ill. Am J Kidney Dis. 2006;48(2):262–268.
- Gettings LG, Reynolds HN, Scalea T. Outcome in post-traumatic acute renal failure when continuous renal replacement therapy is applied early vs. late. Intensive Care Med. 1999;25(8):805–813.
- Bouman CS, Oudemans-Van Straaten HM, Tijssen JG, Zandstra DF, Kesecioglu J. Effects of early high-volume continuous venovenous hemofiltration on survival and recovery of renal function in intensive care patients with acute renal failure: A prospective, randomized trial. Crit Care Med. 2002;30(10):2205–2211.
- Wald R, Deshpande R, Bell CM, Bargman JM. Survival to discharge among patients treated with continuous renal replacement therapy. Hemodial Int. 2006;10(1):82–87.
