Abstract
Background: Referral patterns for palliative medicine consultation (PMC) by intensivists for patients requiring continuous renal replacement therapy (CRRT) have not been studied. Methods: We retrospectively analyzed clinical data on patients who received CRRT in a tertiary referral center between 1999 and 2006 to determine timeliness and effectiveness of PMC referrals and mortality rate as a surrogate for safety among patients receiving CRRT for acute kidney injury. Results: Over one-fifth (21.1%) of the 230 CRRT patients studied were referred for PMC (n = 55). PMC was requested on average after median of 15 hospital and 13 intensive care unit (ICU) days. Multivariate regression analysis revealed no association between mortality risk and PMC. Total hospital length of stay for patients who died after PMC referral was 18.5 (95% CI = 15–25) days compared with 12.5 days (95% CI = 9–17) for patients who died without PMC referral. ICU care for patients who died and received PMC was longer than for patients with no PMC [11.5 (95% CI = 9–15) days vs. 7.0 (95% CI = 6–9) days, p < 0.01]. CRRT duration was longer for patients who died and received PMC referral than for those without PMC [5.5 (95% CI = 4–8) vs. 3.0 (95% CI = 3–4) days; p < 0.01]. Conclusions: PMC was safe, but referrals were delayed and ineffective in optimizing the utilization of intensive care in patients receiving CRRT. A proactive, “triggered” referral process will likely be necessary to improve timeliness of PMC and reduce duration of non-beneficial life-sustaining therapies.
INTRODUCTION
Patients with acute kidney injury (AKI) requiring continuous renal replacement therapies (CRRTs) face staggering risks. The in-hospital mortality of patients with AKI requiring CRRT ranges between 53%Citation1 and 69%.Citation2 Physical symptoms and emotive suffering remain frequent,Citation3,4 affecting patients and family members.Citation5–9 Increased duration of intensive care in non-survivors is associated with decreased quality of life and psychological adjustment among their family members.Citation10
Little is known about the transition from aggressive renal care to a palliative phase. A study of 258 patients with end-stage renal disease (ESRD) contemplating initiation of dialysis reported that median survival among patients who accepted palliative care goals was similar to patients who did not.Citation11 However, in the context of intensive care requiring CRRT, nephrologists and intensivists face more severe dilemmas, which ironically escalate in proportion to the therapeutic advances. Patients with AKI, like other critically ill patients, face relatively limited chances of recovery. However, unlike the general intensive care unit (ICU) population, patients with AKI can be treated with CRRT, one of the potentially most successful medical therapies. This dynamic of realistic hope facing highly unfavorable prognosis distinguishes patients receiving CRRT from other populations previously studied.Citation12–16 While palliative care guidelines for chronic renal disease exist,Citation17,18 literature exploring the impact and timing of palliative medicine referrals in patients undergoing CRRT is lacking.
Emerging consensusCitation19 and research reportsCitation20,21 support integration of palliative and intensive care. In recent years, inpatient palliative care services have become common in US hospitals.Citation22 Palliative medicine consultations (PMC) have been associated with decreased medical ICU (MICU) stay for a broad range of high-risk patients.Citation12,13 Adding complexity, however, is the challenge from the lay press regarding the relative safety of palliative careCitation23 in light of reports that decisions to limit life-sustaining therapies were shown to increase 60-day mortality after matching for disease severity.Citation24 Under experimental conditions, proactively requested palliative and ethics consultations were safe, while reducing non-beneficial ICU utilization and length of stay (LOS).Citation12,25–29 Yet intensivists’ referral patterns for PMC and their outcomes in routine critical renal care remain uncertain.
In this retrospective analysis, we investigated three Institute of Medicine (IOM) consensus-derived characteristics for PMC referral in patients receiving CRRT for AKI in a single tertiary referral hospital: (1) timeliness, (2) mortality as a surrogate for safety, and (3) effectiveness. According to IOM definitions, timeliness aims at reducing waits and delays in needed care. Optimal survival and its inverse, mortality, are metrics of safety. Effectiveness focuses on providing services only to those who could benefit.Citation16 Analyses of intrinsic PMC characteristics, ICU culture, and interprofessional collaboration, or the rationale for PMC referral used by intensivists, were outside of the scope and design of this project.
MATERIALS AND METHODS
Environment
St. Joseph’s Hospital (SJH) is a rural tertiary teaching hospital with over 500 licensed beds and a certified level II trauma center with Magnet designation. Census of individual admissions is approximately 18,000 per year (range 17,946–18,649, during years 2003–2006). Adult patients may have CRRT ordered in either the 21-bed surgical ICU (SICU) or a 16-bed MICU. Both units are staffed by specialists in surgical or medical intensive care and by residents. No formal policies or protocols mandate specific schedules or formats for communicating with patients and families about prognosis or life-sustaining treatments. Prognostic scores, such as Cleveland Clinic Foundation (CCF) or Acute Physiology and Chronic Health Evaluation (APACHE), are not systematically calculated to guide individual clinical decisions and communication. There are no policies, protocols, or formal “triggers” governing initiation of PMC in the ICUs. Rather, referrals are made at the discretion of intensivists whenever deemed clinically appropriate.
PMCs, offered at SJH since 1999, are available 24 h/day, 365 days/year throughout the hospital, including both ICUs. Principal palliative care is provided in a dedicated, 11-bed inpatient palliative care unit. Three of the five consultant physicians are board-certified palliative specialists who dedicate full-time effort (FTE) to the PMC, with a fourth member contributing an additional 0.25 FTE. Fellows in training have served in a supervised, fifth physician-consultant capacity since 2003. Allied services, including social work and chaplaincy, are available at all times for ICU patients and families. Additional materials and methods are described in the Appendix.
Study Design and Patient Sample
After approval by the Institutional Review Board, this retrospective cohort study analyzed PMC referral characteristics for patients treated with CRRT at SJH between January 1999 and February 2006. This study was a substudy within a broader initiative examining CRRT outcomes (See ).
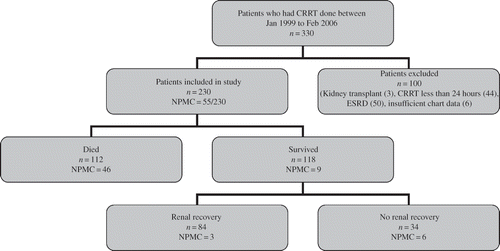
Figure 1. Flow diagram of the participants into the study; PMC distribution (n = 55). Mean age = 66.0 years (±14.5, median 68.8). Males = 61.3%. Females = 38.7%. Mean BUN before CRRT = 63.6 ± 34.2 mg/dL (median 55.5 mg/dL). Mean creatinine before CRRT 3.7 ± 1.8 mg/dL (median 3.5 mg/dL). Mean CCF score at CRRT initiation = 9.12 ± 2.8 (median 9). BUN, blood urea nitrogen; CRRT, continuous renal replacement therapy; NPMC, number of patients included in the palliative referral study; CCF, Cleveland Clinic Foundation; PMC, palliative medicine consultation.
Inclusion Criteria
| 1. | Diagnosis of AKI (creatinine ≥2.0 mg/dL if admission creatinine <1.4 mg/dL or creatinine ≥2.5 mg/dL if admit creatinine >1.5 mg/dL); | ||||
| 2. | admission to MICU or SICU; | ||||
| 3. | CRRT (continuous veno-venous or arterio-venous hemodialysis/filtration) >24 h in duration. | ||||
Exclusion Criteria
| 1. | Renal transplant; | ||||
| 2. | ESRD, on dialysis; | ||||
| 3. | CRRT received less than 24 h; | ||||
| 4. | CRRT received for other than AKI. | ||||
Study Protocol and Data Collection
Data were abstracted from the ICU daily care nursing records by Marshfield Clinic Research Foundation clinical research coordinators trained in data abstraction. Data abstraction was quality assured by applying a 10% reabstraction to randomly selected records early in the abstraction process to ensure accuracy of the abstracted data.
Definitions of Outcomes Studied
Timeliness
Timeliness of PMC referral was examined for evidence of delays in initiating palliative support. Following proactive MICU protocols utilized in successful interventions,Citation9,12,13 we defined timely PMC referral as order entry for formal, specialist physician-level consultation within 5 days from the time of CRRT initiation. Time between hospital, ICU, and PM admission was recorded.
Timeliness of PMC referral was further stratified according to initial CCF scores, which were applied to group patients into two risk categories. The CCF score was developed by Paganini et al.Citation30 as a predictive indicator for mortality and was calculated in our sample 24 h prior to CRRT initiation and on the day of PMC. For this analysis, an initial CCF score of 12 was selected to compare PMC timeliness in patients with the highest initial likelihood of death (exceeding 70%, based on the original Cleveland Clinic mortality data) to those with lesser initial risk (CCF < 12), where 12 represented the median CCF score for the high-risk group (CCF range = 8–14).Citation30
Mortality
To evaluate mortality rate relative to PMC referral, a multivariate analysis of pertinent physiological and clinical parameters was performed. Abstracted variables included all parameters originally evaluated when the CCF score was originally defined.Citation30
Effectiveness
Following IOM consensusCitation16 and the definition of Schneiderman et al.,Citation26 effectiveness of PMC referral was defined as refraining from continuing CRRT and life-sustaining measures in patients who ultimately did not benefit. Occurrence and duration of non-beneficial life-sustaining treatment (NBLST) (duration of CRRT and total ICU days in non-survivors) was compared in patients with and without PMC referrals. We also compared total hospital days, ICU, and CRRT days among patients with and without PMC who had no renal recovery despite CRRT.
Statistical Analysis
Baseline characteristics were reported using descriptive statistics including mean, median, standard deviation, frequency, and percentage. Survival time comparison was conducted using survival analysis. Comparisons for discrete variables were performed using chi-square or Fisher’s exact tests. Comparisons for continuous outcomes were performed using Wilcoxon rank sum test or t-test. Univariate analyses including logistic regression analysis; chi-square, Fisher’s exact, and t-tests; and Wilcoxon rank sum test were applied to identify significant risk factors for mortality. Multivariate logistic regression analysis with backward elimination was used to define determinant risk factors.
RESULTS
Patients (n = 330) who underwent CRRT from January 1999 to February 2006 at ICUs of SJH were evaluated for inclusion in this study, and 230 met inclusion criteria. The remaining 100 patients met the following exclusion criteria: history of kidney transplant (n = 3), history of ESRD (n = 50), duration of CRRT less than 24 h (n = 44), or incomplete data in their medical record (n = 6). Data on all 55 patients who received PMC and CRRT during the defined interval were included in subsequent analyses (See ). Baseline demographic and physiologic data are presented in . Complete acuity, etiology, and outcome data on our sample are described elsewhere.Citation31
Table 1. Baseline patient demographics and clinical characteristics.
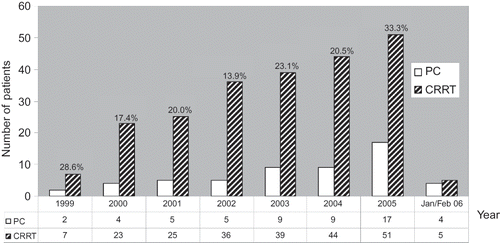
The following PMC utilization rate and outcomes were observed among patients receiving CRRT during the observational period: 55/230 (23.9%) who received CRRT received PMC; 46/55 died while 9/55 was discharged alive; 6/55 had renal recovery while 49/55 did not. In addition to the initial CCF score, follow-up CCF scores were calculated for 43/55 patients with sufficient data on record, derived within 24 h preceding PMC. Patients who were referred for PMC and survived had more favorable follow-up CCF scores than those who died, despite identical initial scores (). Annual trends in absolute numbers of PMC and fraction of PMC among all patients who received CRRT are presented in .
Table 2. Initial and follow-up (pre-PMC referral) CCF scores.
Figure 2. Annual trends in CRRT and PMC between 1999 and February 2006, percentages denote the proportion of all CRRT patients referred for PMC in a given year. CRRT, continuous renal replacement therapy; PMC, palliative medicine consultation.
Timeliness
Elapsed Time and Duration of CRRT Prior to PMC
One-third (34.5%) of PMC referrals (19/55) occurred within 5 days following CRRT initiation. Among all PMC patients, median elapsed time between initiation of CRRT and PMC referral was 9 days. Median duration of CRRT prior to the referral was 5 days. Thirteen CRRT patients with highest initial mortality risk (initial CCF score ≥ 12) had PMC referral sooner than the 42 with CCF < 12 (). Differences between groups relative to PMC referral did not achieve statistical significance.
Table 3. Treatment duration according to median initial CCF scores.
Hospitalization and ICU Days Prior to PMC
For CRRT patients with PMC referrals (n = 55), median admission-to-PMC (A-PMC) interval was 15 days, while median time from initiation of ICU care to PMC was 13 days ().
Post hoc analysis relative to mortality outcome () showed that patients discharged alive tended to receive earlier PMC referrals during hospitalization, experience shorter duration of CRRT, and have shorter ICU stays than those who died.
Table 4. Length of hospitalization and ICU days prior to PMC relative to mortality outcomes.
Mortality
Application of a multivariable logistic regression model defined factors associated with in-hospital mortality (). Good model calibration and fit were achieved (AuROC curve: c = 0.948; Hosmer–Lemeshow goodness-of-fit test: χ2 = 4.45, DF = 7; p = 0.73). PMC referral was not an independent mortality risk factor.
Table 5. Multivariate logistic regression analysis of mortality factors among all CRRT patients.
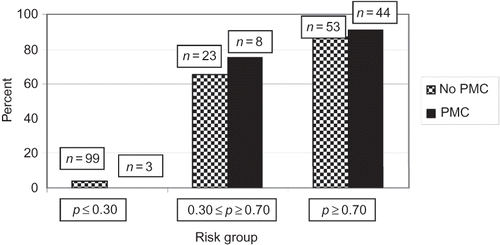
The predictive probabilities of death were calculated and stratified to three groups (low risk = p < 0.30; medium risk = 0.30 < p < 0.70; high risk = p > 0.70). Strata were used to evaluate the relationship between PMC and death. At each stratum of risk, the difference in mortality between PMC and no PMC was not significant; (Fisher’s exact test p-value was close to 1.000) ().
Figure 3. Mortality of patients with and without PMC within risk strata. p-Values for all three strata = 1.0 (Fisher’s test). PMC, palliative medicine consultation.
The following descriptive data characterized patients with a “Do Not Resuscitate” (DNR) order which was a mortality factor. There were DNR orders for 51/175 (29.14%) with no PMC and 49/55 (89.09%) with PMC. Among patients with DNR orders and PMC, 27/49 (55.1%) patients had DNR orders prior to PMC. A median of 3 days (mean = 5.3) elapsed between the DNR and PMC for those patients. DNR orders were placed on the day of PMC for 20/49 (40.8%) patients or following PMC for 2/49 (4.1%) patients.
Effectiveness
Total Duration of Non-Beneficial Life-Sustaining Treatments
Patients referred for PMC who died received significantly more intensive and longer care overall than those with no PMC (). CRRT treatment days for patients with PMC referral preceding death (5.5; 95% CI: 4–8) were longer than for patients who died with no PMC (3.0; 95% CI: 3–4). ICU LOS and total hospital LOS for patients with PMC who died were also longer than in the non-referred patients (). In contrast, intensity and duration of life-sustaining care were indistinguishable among surviving patients, whether they received PMC or not ().
Table 6. Non-beneficial life-sustaining treatments (NBLST): CRRT, ICU, and total hospital days among all deceased patients.
Duration and Intensity of Care Among Patients with Renal Non-Recovery
Patients with no renal recovery referred for PMC experienced longer and more intensive care than non-referred patients. Duration of CRRT was 66% longer among patients with PMC referral (5 vs. 3 days, p < 0.01), ICU stay was 43% longer (10 vs. 7 days, p < 0.01), while total hospital LOS was 36% longer (19 vs. 14 days, p = 0.01).
Intensive Care Duration Prior to PMC Referral Based on Survival
Patients with PMC referral who ultimately died were hospitalized longer prior to PMC and received more CRRT days than those who survived. Median A-PMC interval for surviving patients with PMC (n = 9) was 8 days, but was 16.5 days for patients who died (n = 46) (). However, there was no statistically significant difference in the A-PMC interval between patients who lived and those who died. Patients who received PMC and ultimately died underwent CRRT for a longer period than those who survived to discharge while receiving palliative support (median CRRT–PMC = 5.5 vs. 3 days, p = 0.28). In contrast, patients who survived with or without PMC experienced comparable total hospital LOS, duration of CRRT, ICU care, and initial CCF scores ().
Table 7. Comparison of patients with and without PMC who survived.
Table 8. CRRT utilization among patients with and without PMC referral.
Chronology of Care: Efficiency versus Timeliness
While the patients with higher initial CCF score had higher mortalityCitation31 and received PMC earlier than those with lower CCF (), when comparing all patients who died, those with PMC referral experienced significantly more NBLST. This finding was counterintuitive, since one expects less NBLST with earlier referral of high-risk patients. These data prompted charting of chronology of nodal care events among all patients who died (). For non-survivors with no PMC, median CRRT duration was 3 days and median hospital LOS was 12.5 days. By contrast, median hospital LOS prior to PMC (A-PMC) for non-survivors was 16.5 days, median CRRT duration was 5.5 days, median period from CRRT to PMC was 10 days, and median (total) hospital LOS was 18.5 days (). Notably, likelihood of mortality based on initial CCF scores did not differ among non-survivors irrespective of PMC status (median CCF = 10, both groups).
Figure 4. Chronology of palliative medicine consultation (PMC) referrals in relation to other sentinel events [total hospital length of stay, total continuous renal replacement therapy (CRRT) days only, number of days from initiation of CRRT until PMC referral, number of days in intensive care until PMC referral].
![Figure 4. Chronology of palliative medicine consultation (PMC) referrals in relation to other sentinel events [total hospital length of stay, total continuous renal replacement therapy (CRRT) days only, number of days from initiation of CRRT until PMC referral, number of days in intensive care until PMC referral].](/cms/asset/96dc6853-c638-43de-a0cf-8ea63699977b/irnf_a_589946_f0004_b.gif)
DISCUSSION
A transition from curative to palliative phase of care occurs for approximately half of the CRRT-treated patients. At our institution, formal, around-the-clock, physician-specialist, palliative care has been available for more than a decade, with over 100 ICU-based consultations received annually. Given this long history, capacity, and integration of palliative care into the workflow of intensive care, we anticipated a pattern of early referral for PMC, with decreased duration of non-beneficial treatments and no increase in mortality in association with PMC.
Unexpectedly, this retrospective analysis found a pattern of delayed PMC referrals, particularly for patients who ultimately died. Among the entire cohort of 230 patients, CRRT was discontinued for the majority of patients on the third CRRT day (median), at which time most of the patients either improved or died. PMC was requested on the ninth day (median) post-initiation of CRRT for the minority of patients who neither improved nor died during the 5 days of CRRT and the additional 4 days off CRRT (), as evidenced by the median CRRT-to-PMC interval of 9 elapsed days. Similarly, median hospital days to PMC (n = 15) were almost 3 days longer than the total hospital LOS for patients who died without receiving PMC. This pattern suggests that most PMC referrals were generated in reaction to patients’ persistently ambivalent clinical status. Among patients who died, a majority (n = 66) declined rapidly, and without benefit of PMC referral. The remaining non-survivors (n = 46) who had PMC referral exhibited protracted clinical decline. However, for these patients referrals were delayed. It is thus likely that a long LOS, rather than objective patients’ needs, functions as a referral trigger.Citation32
The retrospective design notwithstanding, our study confirmed the findings of Norton et al.Citation12 who demonstrated no additional mortality associated with a proactive PMC in the MICU setting. In this study, multivariate analysis did not detect PMC as a significant risk factor for death. Further, Fisher’s exact test p-value for each stratum of mortality risk was close to 1.000, supporting the safety of PMC. However, the presence of a DNR order, most frequently placed by ICU physicians (median on day 3) prior to PMC referral, was the only decision-contingent, statistically significant, mortality-associated factor, confirming existing research data.Citation24
We anticipated increased effectiveness of care associated with PMC referrals as previously demonstrated by Norton et al.Citation12 In this study, applying the definition of Schneiderman et al.,Citation26 effectiveness of PMC referral was narrowly defined as prevention (reduction) of “non-beneficial life-sustaining treatments.” Unexpectedly, we found that for most subgroups of patients, PMC referral was associated with excess utilization of all studied parameters of non-beneficial treatments: duration of CRRT, ICU, and total hospital LOS among patients who ultimately died. These findings were likely attributable to the relatively late, reactive PMC referral pattern, almost certainly introducing a late referral bias. Such timing made meaningful decreases in non-beneficial treatments unlikely.
We determined that a median of only 3 days of CRRT support was received by 175/230 patients (76%) (). For the remaining patients who neither improved nor died, a median of an additional 6 days elapsed until PMC referral. When patients were stratified a posteriori to high versus low CFF score, patients with lower risk were found to have much later referral () than those with the highest mortality risk. Exact reasons for this delay in PMC cannot be ascertained due to the retrospective study design. However, tension between timeliness and effectiveness of the palliative referrals on the one hand and their safety on the other hand must be recognized. Theoretically, excessive emphasis on timeliness could lead to premature referrals and excess mortality, thus undermining safety. In the absence of reliable prognostic models,Citation33 prospective objective discrimination between individual survivors versus non-survivors of AKI requiring CRRT remains impossible. Prognostic performance and limitations of the CCF model in our sample is described elsewhere.Citation31 Furthermore, when presented with identical clinical scenarios, intensivists’ mortality predictions differ, suggesting that personal perceptions impact clinical impressions and willingness to continue intensive support.Citation34 Given that some of the critical decisions customarily discussed in the course of PMC, such as withdrawal of life-sustaining support, may be irreversible and associated with post-decisional regret, some caution about safety of PMC referral might appear justified. However, in order to be cogent, such caution would have to be predicated on an improper belief that PMC causes premature termination of CRRT. Yet, the absence of excess mortality associated with PMC has been demonstrated in our cohort and in previous studies.Citation12,26,27 Indeed, in a corollary finding, we observed that among all patients who were referred for PMC, the survivors received palliative consultations earlier during their hospitalization than those who died. Therefore, a far more likely consequence of delayed PMC was accrual of the excess discomfortCitation10 and non-beneficial treatments. Rather than striving for an ideal prognostic positive predictive value prior to PMC referral, we suggest the utilization of early structured communication about patients’ individual values, while acknowledging a dynamic equipoise of individual versus community interests, notably, competing demands of beneficence and justice.
The retrospective, single-institution study design limits the scope of our conclusions. However, comparing our administrative data to that compiled from the Palliative Care Leadership Centers,Citation35 it appears that the total number of palliative “patient cases” at SJH already exceeds those at the leading institutions, while the duration of palliative care support for individual patients is similar (mean of 5.5 days at SJH vs. 6.5 days elsewhere).Citation35 The mean LOS for patients who received PMC in our study (n = 26) was identical to that reported from two large hospitals in a different geographic region.Citation36 Therefore, the delays in PMC referrals that we documented are as likely to occur elsewhere. Second, the apparent referral delay may reflect intensivists’ utilization of their own state-of-the-art palliative skills. If this is the case, only the most complex patients are referred for PMC, with a justifiable time lag. However, such exemplary competence was not self-reported in a recent national survey of intensivists.Citation37 Third, the long interval between initiation of CRRT and PMC referral may reflect unusually early initiation of CRRT rather than delay in the referral. Such lead time bias, however, appears unlikely given that the nephrologists broadly conform to evidence-based, standardly applied renal indications for the therapy. Fourth, neither the intensivists’ communication strategies with patients and families nor demographics and the cultural context of the ICU can be determined from our study due to its retrospective design. These factors impact timeliness and effectiveness of PMC referralsCitation38 by introducing confounders.Citation39 However, the interval between ICU admission and PMC in our sample (13 days) was very similar to that reported from the two largest routine PMC in ICU samples reported to date (10 daysCitation40 to 14 daysCitation12). This suggests that our observations about timeliness and efficiency of PMC referrals in CRRT may reflect similar delays elsewhere, regardless of the local ICU cultural and demographic characteristics.
Although palliative care services are available in 75.4% of large US hospitals,Citation41 proactive PMC for patients with AKI has not been integrated into standard clinical care. Despite advanced planning and availability of excellent clinical care and service integration, many patients and families choose prolonged intensive renal support that ultimately fails and is associated with increased duration of non-beneficial treatments, as noted in our study. Our observations strongly support future controlled studies of proactive protocols for patient-centered transition from curative to palliative phases of care. Such transitioning requires systems and processes that integrate operator-independent referral mechanisms into the present model of intensive care. PMC might be ordered as early as on the third day of CRRT (certainly for all patients who are considered by the attending ICU physicians for a DNR order), show no evidence of renal recovery, and/or exhibit accelerating physiological indices of illness severity. Importantly, all of these indicators of high risk appear statistically independent and additive.
CONCLUSION
Availability of palliative care well integrated into ICU care alone is not sufficient for best intensive renal care. We observed prolonged utilization of non-beneficial treatments that carry a previously demonstrated risk of excessive symptom burden for patients and their families. Given our findings, timeliness and effectiveness of palliative referrals for patients with AKI requiring CRRT are more likely to be realized with proactive consultations triggered by meeting preset clinical conditions.
ACKNOWLEDGMENTS
The authors thank the Marshfield Clinic Research Foundation’s Office of Scientific Writing and Publication for assistance in the preparation and critical review of this manuscript.
Funding. Funding for this study was provided by the Marshfield Clinic, Physician Research Funds.
Financial Conflict of Interest: Authors are employees of Marshfield Clinic. Authors report no real or perceived conflict of interest.
REFERENCES
- Swartz R, Perry E, Daley J. The frequency of withdrawal from acute care is impacted by severe acute renal failure. J Palliat Med. 2004;7(5):676–682.
- Augustine JJ, Sandy D, Seifert TH, Paganini EP. A randomized controlled trial comparing intermittent with continuous dialysis in patients with ARF. Am J Kidney Dis. 2004;44(6):1000–1007.
- Desbiens NA, Wu AW, Broste SK, Pain and satisfaction with pain control in seriously ill hospitalized adults: Findings from the SUPPORT research investigations. For the SUPPORT investigators. Study to understand prognoses and preferences for outcomes and risks of treatment. Crit Care Med. 1996;24(12):1953–1961.
- Nelson JE, Meier DE, Oei EJ, Self-reported symptom experience of critically ill cancer patients receiving intensive care. Crit Care Med. 2001;29(2):277–282.
- Azoulay E, Pochard F, Kentish-Barnes N, Risk of post-traumatic stress symptoms in family members of intensive care unit patients. Med Am J Respir Crit Care Med. 2005;71(9):987–994.
- Abbott KH, Sago JG, Breen CM, Abernethy AP, Tulsky JA. Families looking back: One year after discussion of withdrawal or withholding of life-sustaining support. Crit Care Med. 2001;29(1):197–201.
- Kris AE, Dodd MJ. Symptom experience of adult hospitalized medical-surgical patients. J Pain Symptom Manage. 2004;28(5):451–459.
- Murtagh FE, Addington-Hall JM, Edmonds PM, Symptoms in advanced renal disease: A cross-sectional survey of symptom prevalence in stage 5 chronic kidney disease managed without dialysis. J Palliat Med. 2007;10(6):1266–1276.
- Lautrette A, Darmon M, Megarbane B, A communication strategy and brochure for relatives of patients dying in the ICU. N Engl J Med. 2007;356(5):469–478.
- Wright AA, Zhang B, Ray A, Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300(14):1665–1673.
- Smith C, Da Silva-Gane M, Chandna S, Warwicker P, Greenwood R, Farrington K. Choosing not to dialyse: Evaluation of planned non-dialytic management in a cohort of patients with end-stage renal failure. Nephron Clin Pract. 2003;95(2):c40–c46.
- Norton SA, Hogan LA, Holloway RG, Temkin-Greener H, Buckley MJ, Quill TE. Proactive palliative care in the medical intensive care unit: Effects on length of stay for selected high-risk patients. Crit Care Med. 2007;35(6):1530–1535.
- Campbell ML, Guzman JA. Impact of a proactive approach to improve end-of-life care in a medical ICU. Chest. 2003;123(1):266–271.
- Campbell ML, Guzman JA. A proactive approach to improve end-of-life care in a medical intensive care unit for patients with terminal dementia. Crit Care Med. 2004;32(9):1839–1843.
- Smith TJ, Coyne P, Cassel B, Penberthy L, Hopson A, Hager MA. A high-volume specialist palliative care unit and team may reduce in-hospital end-of-life care costs. J Palliat Med. 2003;6(5):699–705.
- Committee on Quality of Health Care in America. Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Available at: http://www.nap.edu/catalog.php?record_id=10027#toc. Accessed February 12, 2009.
- Cohen LM, Moss AH, Weisbord SD, Germain MJ. Renal palliative care. J Palliat Med. 2006;9(4):977–992.
- Moss AH. Shared decision-making in dialysis: The new RPA/ASN guideline on appropriate initiation and withdrawal of treatment. Am J Kidney Dis. 2001;37(5):1081–1091.
- Clarke EB, Curtis JR, Luce JM, Quality indicators for end-of-life care in the intensive care unit. Crit Care Med. 2003;31(9):2255–2262.
- Mosenthal AC, Murphy PA. Interdisciplinary model for palliative care in the trauma and surgical intensive care unit: Robert Wood Johnson foundation demonstration project for improving palliative care in the intensive care unit. Crit Care Med. 2006;34(11 Suppl.):S399–S403.
- Ray D, Fuhrman C, Stern G, Integrating palliative medicine and critical care in a community hospital. Crit Care Med. 2006;34(11 Suppl.): S394–S398.
- Morrison RS, Maroney-Galin C, Kralovec PD, Meier DE. The growth of palliative care programs in United States hospitals. J Palliat Med. 2005;8(6):1127–1134.
- Allen C. Back Off! I’m not Dead Yet. Washington Post. October 14, 2007:B01.
- Chen YY, Connors AF Jr, Garland A. Effect of decisions to withhold life support on prolonged survival. Chest. 2008;133(6):1312–1318.
- Morrison RS, Penrod JD, Cassel JB, Cost savings associated with US hospital palliative care consultation programs. Arch Intern Med. 2008;168(16):1783–1790.
- Schneiderman LJ, Gilmer T, Teetzel HD, Effect of ethics consultations on nonbeneficial life-sustaining treatments in the intensive care setting: A randomized controlled trial. JAMA. 2003;290(9):1166–1172.
- Temel JS, Greer JA, Muzikansky A, Early palliative care for patients with metastaic non-small-cell lung cancer. N Engl J Med. 2010;363(8):733–742.
- Kelley AS, Meier DE. Palliative care—A shifting paradigm. N Engl J Med. 2010;363(8):781–782.
- Germain MJ, Kurella Tamura M, Davison SN. Palliative care in CKD: The earlier the better. Am J Kidney Dis. 2011;57(3):378–380.
- Paganini EP, Halstenberg WK, Goormastic M. Risk modeling in acute renal failure requiring dialysis: The introduction of a new model. Clin Nephrol. 1996;46(3):206–211.
- Vats H, Dart R, Okon T, Liang H, Demijian S, Paganini E. Continuous renal replacement therapy: Analysis of experience and outcomes in a tertiary care center. Ren Fail. ( manuscript submitted 2011).
- Smith TJ, Cassel JB. Cost and non-clinical outcomes of palliative care. J Pain Symptom Manage. 2009;38(1):32–44.
- Sinuff T, Adhikari NK, Cook DJ, Mortality predictions in the intensive care unit: Comparing physicians with scoring systems. Crit Care Med. 2006;34(3):878–885.
- O’Brien JM Jr, Aberegg SK, Ali NA, Diette GB, Lemeshow S. Results from the national sepsis practice survey: Predictions about mortality and morbidity and recommendations for limitation of care orders. Crit Care. 2009;13(3):R96.
- America’s Care of Serious Illness. A State-by-State Report Card on Access to Palliative Care in Our Nation’s Hospitals. Center to Advance Palliative Care. National Palliative Care Research Center. Available at: http://www.capc.org/reportcard/findings. Accessed February 12, 2009.
- Penrod JD, Deb P, Luhrs C, Cost and utilization outcomes of patients receiving hospital-based palliative care consultation. J Palliat Med. 2006;9(4):855–860.
- Nelson JE, Angus DC, Weissfeld LA, End-of-life care for the critically ill: A national intensive care unit survey. Crit Care Med. 2006;34(10):2547–2553.
- Baggs JG, Norton SA, Schmitt MH, Dombeck MT, Sellers CR, Quinn JR. Intensive care unit cultures and end-of-life decision making. J Crit Care. 2007;22(2):159–168.
- Starks H, Diehr P, Curtis JR. The challenge of selection bias and confounding in palliative care research. J Palliat Med. 2009;12(2):181–187.
- Delgado-Guay MO, Parsons HA, Li Z, Palmer LJ, Bruera E. Symptom distress, interventions, and outcomes of intensive care unit cancer patients referred to a palliative care consult team. Cancer. 2009;115(2):437–445.
- Siu AL, Spragens LH, Inouye SK, Morrison RS, Leff B. The ironic business case for chronic care in the acute care setting. Health Aff (Millwood). 2009;28(1):113–125.
- Cohen LM, Germain MJ, Poppel DM. Practical considerations in dialysis withdrawal: “To have that option is a blessing”. JAMA. 2003;289(16):2113–2119.
- National Consensus Project for Quality Palliative Care. Available at: http://www.nationalconsensusproject.org/Guidelines_Download.asp. Accessed February 12, 2009.
- Renal Physicians’ Association (RPA). Shared Decision-Making in the Appropriate Initiation of and Withdrawal from Dialysis. 2nd ed., Rockville, MD: Renal Physicians’ Association; 2010.
APPENDIX. ADDITIONAL MATERIALS AND METHODS
There were more than 600 palliative medicine consultations (PMCs) annually at St. Joseph’s Hospital (range 665–754 during the 5-year study period). PMCs requested by intensivists exceeded 100/year (range 108–136) during the study interval. The relative frequency of all PMC as a fraction of all hospital discharges (PMC/discharges, annualized = “patient cases”Citation25) at SJH ranges from 3.7% to 4.2%. The structure and process of PMC adhere to the published clinical practice guidelines for patients in transition from aggressive renal replacement therapy to palliative care,Citation18,41–44 emphasizing informed, shared decision making. Most of the patients who receive PMC are discharged alive. While a substantial minority of these patients enroll in hospice care following discharge, the majority continue to receive either restorative or palliative support. Principal care of patients who elect comfort or palliative goals is customarily transferred to palliative care physicians, usually in the setting of the palliative care unit.
Since SJH and Marshfield Clinic share a comprehensive and sophisticated combined electronic medical record (EMR) dating back to the 1960s that can be readily interrogated for laboratory data, study data were abstracted from the EMR. Data in the EMR are captured in a Datawarehouse that captures and comprehensively backs up all data daily in real time. An extensive database was populated for the retrospective CRRT cohort that captured data on demographics (gender, age, admission dates), CRRT (time of onset, duration, vascular access, anticoagulation, complications), cause of acute kidney injury, comorbidities, multisystem involvement, and survival and renal recovery status for the index hospitalization.
